Peripartum cardiomyopathy (PPCM) is defined as the development of an idiopathic cardiomyopathy presenting with heart failure secondary to left ventricular systolic dysfunction, towards the end of pregnancy or in the months following birth, where no other cause of heart failure is found. All other possible causes of heart failure, for example coronary heart disease, myocarditis, must be excluded before the diagnosis can be made. The left ventricle is not always dilated but the ejection fraction is always reduced below 45% (Sliwa et al, 2010).
PPCM is difficult to diagnose because breathlessness, reduced exercise tolerance and feeling short of breath lying flat are not uncommon in normal pregnancy. The diagnosis of PPCM therefore relies on awareness of the condition by medical professionals, who must appreciate that progressive symptoms are abnormal and investigate appropriately. If PPCM is suspected this should prompt immediate referral to a cardiologist with urgent chest X-ray and echocardiography.
Epidemiology
The most recent prevalence data of PPCM in the UK has been estimated from maternal mortality and morbidity data, in the period 2009–12 from the Centre for Maternal and Child Enquiries. Maternal deaths have decreased from 11/100 000 (2006–08) to 10/100 000 (2010–12) of women giving birth. Of these, 68% of women died from indirect causes, such as PPCM, and only 32% died from direct complications such as bleeding (Cantwell et al, 2011; Freedman and Lucas, 2015). Reports estimate an incidence of between 1 case per 15 000 live births in the US (Desai et al, 1995; Chapa et al, 2005; Fett, 2005; Brar et al, 2007) to 1 case per 1000 live births in South Africa and 1 case per 300 live births in Haiti (Fett, 2005). There is a disproportionately high incidence in certain ethnic groups, such as those of African descent, and a lower incidence in Hispanics (Brar et al, 2007). A high prevalence in Nigeria is caused by the tradition of ingesting kanwa (dried lake salt) for 40 days postpartum, which leads to volume overload. Seventy-five percent of cases are diagnosed within the first postpartum month, and around 45% of these present in the first week (Sliwa et al, 2010).
The incidence of PPCM overall may be increasing. In their US study, Mielniczuk et al (2006) found an increase from 1:4350 during 1990–1993 to 1:2229 during 2000–2002. Proposed theories for this finding include increasing maternal age, an increasing number of multiple pregnancies, and increased recognition of PPCM (Elkayam, 2011). Enhanced awareness has been promoted by the European Society of Cardiology (ESC), as part of the EURObservational Research Programme (Sliwa et al, 2014).
Morbidity and mortality
The 2-year mortality rate in the US among African American women is 15.9%, while in South Africa it is 10–27% at 6 months and in Haiti 15% at 2 years (Fett, 2005; Brar et al, 2007; Sliwa and Böhm, 2014). In the period 2009–2012, 357 women in the UK died during, or within, 6 weeks of the end of their pregnancy. This represents a statistically significant decrease in the maternal mortality rate, which are 10/100 000 pregnancies. However, maternal deaths from indirect causes, which include peripartum cardiomyopathy, are still not addressed effectively. Actions are urgently needed to address deaths from indirect causes (Knight et al, 2014). The incidence of thromboembolic events in PPCM is high at around 30% and these women are susceptible to pulmonary thromboemboli, as well as systemic emboli secondary to clot formation in the left ventricle (Heider et al, 1999). Once the diagnosis has been made, all patients should receive therapeutic anticoagulation with low molecular weight heparin when pregnant and warfarin if postpartum.
Aetiology
The cause for PPCM is unknown. A number of theories have been proposed including immune system changes (autoimmunity or dysfunction), myocarditis, fetal genetic abnormalities, increased cardiomyocyte cell death and a familial association between PPCM and familial dilated cardiomyopathy (FDCM) (Suri et al, 2013; Ricke-Hoch et al, 2014). A myocardial inflammatory response with detection of cytokines is common in PPCM (Ricke-Hoch et al, 2014; Sliwa et al, 2014; Sliwa and Böhm, 2014).
Deficiencies in nutritional micronutrients may play a role in the pathogenesis of PPCM. Reduced selenium increases cardiovascular susceptibility to viral infections, hypertension and hypocalcemia (Demakis and Rahimtoola, 1971; Demakis et al, 1971; Melvin et al, 1982). The prevalence of genetic mutations associated with FDCM in patients with PPCM suggests that there may be an overlap in the clinical spectrum of these two diseases (Regitz-Zagrosek et al, 2011; van Spaendonck-Zwarts et al, 2014; Van Tintelen et al, 2014). Hilfiker-Kleiner et al (2007) have suggested that high levels of prolactin might trigger PPCM when they found that the suppression of prolactin release using Bromocriptine (a D2 dopamine-receptor agonist) prevented the onset of disease in animal models. Lkhider et al (2010) and Lampert and Lang (1995) found an association between the use of tocolytic therapies and development of pulmonary oedema in pregnant women, and proposed a link between prolonged tocolysis and PPCM.
Clinical presentation
PPCM is more common in multiparous females and in multiple pregnancies. Patients can present with heart failure, respiratory failure or with a thromboembolic complication such as stroke.
The signs and symptoms may be subtle at first presentation but progressive breathlessness, palpitations, oedema, dry cough and lethargy should all raise the possibility of PPCM and prompt further investigation (Sliwa et al, 2010; Cantwell et al, 2011; Elkayam, 2011; Sliwa et al, 2014).
The New York Heart Association (NYHA) functional class is a subjective estimate of a patient's true functional ability (Table 1) (Rossi, 1967). Several predictors of maternal cardiovascular events risk scores have been developed, of which the CARPREG (Jastrow et al, 2011) risk score is most widely known and used. This risk score has been validated in several studies and is helpful when trying to predict maternal risk. In the CARPREG study, a baseline NYHA class >II, estimated a 27% risk of a maternal cardiovascular event (Siu et al, 2001).
| NYHA class | Level of impairment |
|---|---|
| I | No symptom limitation with ordinary activity |
| II | Ordinary physical activity somewhat limited by dyspnoea (long-distance walking, climbing two flights of stairs) |
| III | Exercise limited by dyspnoea with moderate working (short-distance walking, climbing one flight of stairs) |
| IV | Dyspnoea at rest or with little exertion |
The severity of heart failure signs and symptoms depends on the degree of depression of left ventricle systolic function. Those with mild heart failure symptoms who are NYHA class I or II, may just be a little breathless when walking with a dry cough lying flat, in which case they may still contemplate a vaginal birth. For those with more severe heart failure symptoms who are NYHA class III or IV, they usually have overt breathlessness at rest, are tachycardic and have paroxysmal nocturnal dyspnoea, with radiographic alveolar oedema on chest radiograph thereby necessitating operative delivery. The clinical findings include an increased respiratory rate, tachycardia, and raised jugular venous pressure. The oxygen saturations may be normal or low, the latter a late finding and a critical stage of the disease presentation. The chest auscultation may detect crackles or may be clear and it is only the chest radiograph which can confirm or refute the presence of pulmonary oedema.
Investigations
The majority of specialist cardiac investigations will be requested by the physician. However, midwives play an important role at the bedside. Regular careful observations can detect early demise. Cardiac monitoring is essential. Blood pressure should be measured non-invasively until arterial catheters are placed. Venous access should be obtained early to administer medication promptly and draw blood for laboratory tests. In antepartum women, fetal heart rate monitoring should be initiated early to detect compromise. Non-pharmaceutical therapy includes restriction of dietary sodium to 2 g sodium per day and fluid restriction to 2 L/day. Bed rest is no longer recommended due to the increased risk of thromboembolism. Instead, the patient should be encouraged to undertake light exercise. Imminent signs and symptoms of decompensated heart failure are hypotension, worsening heart failure, altered mental status, and increased work of breathing (Sliwa et al, 2010).
Laboratory tests
PPCM is a diagnosis of exclusion; therefore, other causes of heart failure must be considered and investigated.
In addition to standard haematological and biochemical tests, thyroid-function should be checked along with inflammatory markers ESR and C-reactive protein to exclude acute myocarditis. NT-proBNP is a non-specific marker for pregnancy complications such as pre-eclampsia, as well as for heart failure and other diseases. It is often markedly elevated in PPCM patients (McMurray et al, 2012). Myocardial infarction or ischaemia should be excluded by measurement of Troponin T. It is often within normal range in PPCM patients (van Spaendonck-Zwarts et al, 2010; Bhattacharyya et al, 2012).
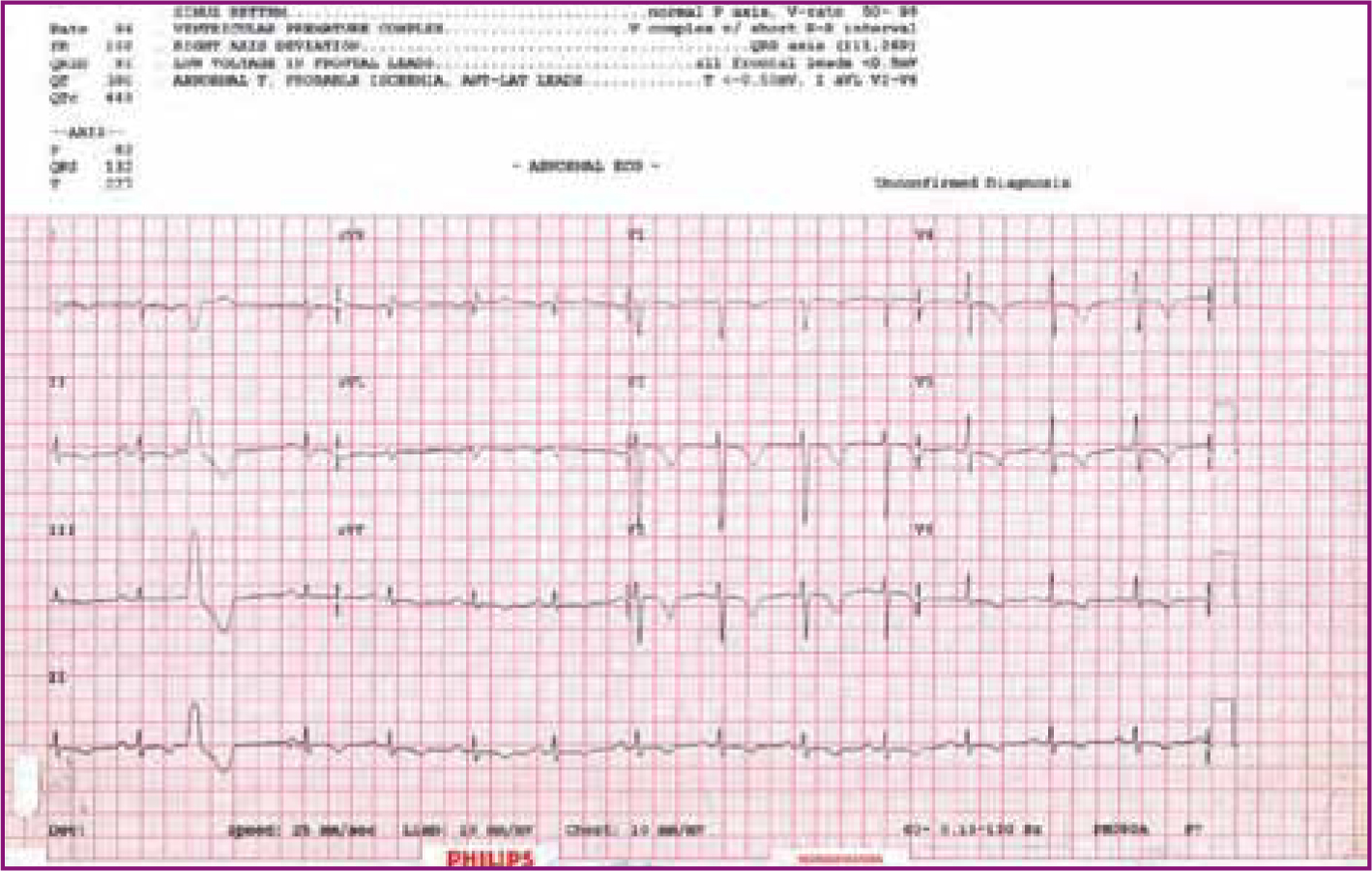
Electrocardiography
An electrocardiograph (ECG) should be performed. The most commonly seen electrocardiographic changes are ST-T wave abnormalities (Figure 1) and left ventricular hypertrophy. Sinus tachycardia is common.
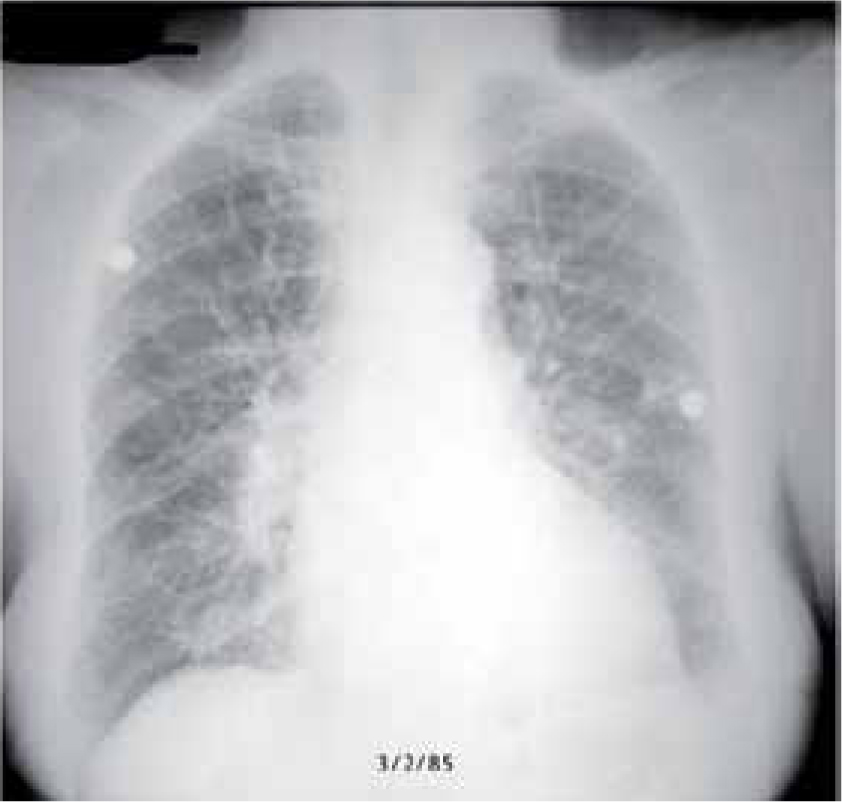
Chest radiography
Chest radiography must be performed. It is safe in pregnancy with a negligible fetal radiation dose. The finding of interstitial or alveolar oedema, with associated cardiomegaly, may confirm the diagnosis of pulmonary oedema (Figure 2).
Transthoracic echocardiography
The diagnostic investigation is transthoracic echo (TTE), which is non-invasive and can be easily performed at the bedside. Initial evaluation should rule out other potential causes of heart failure such as valvular heart disease.
If there is normal left ventricle systolic function, other causes of high output heart failure such as anaemia and thyrotoxicosis should be considered.
There is usually global impairment of left ventricle function in PPCM with depressed ejection fraction of <45%. After diagnosis, TTE surveillance should continue every 4 weeks or so to evaluate the response to medical treatment and monitor the possible recovery of left ventricle function. Postpartum echocardiography is performed before discharge from hospital by cardiologists or cardiac sonographers, and 6 weekly thereafter until left ventricle baseline function is reached, and 6 monthly and annually thereafter.
Magnetic resonance imaging
Magnetic resonance imaging provides additional quantitative assessment of left ventricle function and can detect necrosis and left ventricular thrombi. Gadolinium contrast crosses the placenta, so should be avoided antepartum (Leurent et al, 2009).
Other investigations
Coronary angiography should be considered if there are regional wall motion abnormalities on TTE or acute segmental ST changes on ECG.
Acute treatment
A diagnosis of PPCM is an emergency situation. The patient may become critically ill and within an unpredictable timeframe. Once the diagnosis is made, the patient should be admitted to a high dependency area with facilities for invasive haemodynamic montoring and respiratory support on hand.
The focus of treatment is the mother, not the fetus. Appropriate heart failure treatment should not be restricted in the event of potential harm to the fetus. A care plan should be formulated by a multidisciplinary team, with input from a cardiologist, obstetrician, midwife and neonatologist.
The initial nursing assessment begins with rapid review of vital signs (airway, breathing and circulation). Patients should have continuous ECG and blood pressure monitoring. Intravenous access should be obtained early, so drug therapy can be administered promptly. Fluid balance charts and daily weights must be completed. In antepartum women, early fetal monitoring should be performed as this may detect abnormalities in fetal heart rate indicating poor oxygenation and circulatory compromise, which may prompt early delivery.
The treatment of PPCM is similar to that used in heart failure. Left ventricle pre-load must be reduced by using diuretics, nitrates or hydralazine (McMurray et al, 2012). Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers improve survival in heart failure but they are contraindicated in pregnancy, therefore continuing the pregnancy or interrupting it for the sake of maternal health and prognosis must be factored into treatment decision-making. Hypoxaemia (SpO2<90%) is associated with an increased risk of mortality (McMurray et al, 2012). Oxygen saturations should be kept >95%. Non-invasive and invasive ventilatory support may be necessary in the event of pulmonary oedema, reduced consciousness or physical exhaustion (Dworzynski et al, 2014).
Inotropic support using dobutamine, dopamine and milrinone may be needed to maintain systemic blood pressure and tissue perfusion in the critically sick patient. Levosimendan improves cardiac output and decreases mortality in patients with severely low cardiac ouput; however, it has not been evaluated by randomised controlled trials. Patients should be fully anticoagulated to prevent thromboembolic complications. Low molecular weight heparin is used for the pregnant patient and warfarin postpartum for at least up to 6 weeks. Warfarin is continued long-term if the left ventricular function remains ≤ 30%.
Mechanical support with a balloon pump or ventricular assist device may be necessary. Support is continued until the underlying heart function improves or as a bridge to heart transplantation. Around 25% of patients with PPCM require cardiac transplantation. Rasmusson et al (2007) showed that outcomes of graft failure and death are less for recipients with PPCM, which may be partly explained by younger age, higher allosensitisation, higher pre-transplant acuity, and increased rejection.
Labour and birth
When PPCM is newly diagnosed, continuation of pregnancy and mode of delivery is a decision based on the clinical status of the patient, the degree of left ventricle impairment and the gestational age of the baby. Where possible, pregnancy should continue until fetal viability and/or good fetal outcome can be achieved, but this cannot be at the expense of maternal health. The decision to interrupt a pregnancy is major step and should be made following discussions between the patient and multidisciplinary team.
The mode of birth depends on the clinical condition of the mother and gestational age of the baby. If there is severe heart failure, pulmonary oedema and or an exceptionally pre-term birth, an operative delivery is needed otherwise a vaginal birth may be possible. Effective labour analgesia is important and regional anaesthesia is preferred as it decreases preload and afterload. Both intravenous and local anaesthetic drugs should be carefully titrated, avoiding sudden falls in blood pressure. An anaesthetist with cardiac expertise is required.
Continuous haemodynamic and fluid balance monitoring is recommended.
Postpartum treatment
After birth, angiotensin-converting enzyme inhibitors should be started along with other medications known to improve prognosis in heart failure, including β-blockers. The need for any other device therapies such as implantable cardiac defibrillators or cardiac resynchronisation therapy requires careful consideration as device complications are not uncommon when devices may have to be in-situ for several decades.
There is controversy surrounding breastfeeding. Safirstein et al (2012) showed that breastfeeding mothers fared better, while others have put forward the theory that prolactin metabolites can be cardiotoxic and recommend the use of prolactin inhibitors (Horodnicki et al, 1991; Selle et al, 2009; Sliwa et al, 2010).
Prognosis
The prognosis depends on the recovery of left ventricular function, defined as left ventricular function ≥ 50%, or improvement by >20% (Pieper, 2013). Recovery usually occurs between 3 and 6 months postpartum, but might occur as late as 48 months postpartum (Lampert and Lang, 1995). Delayed diagnosis, poor functional class, Black ethnicity, left ventricle thrombus, multiparity and co-morbidities are associated with delayed recovery (Amos et al, 2006). Women exhibiting low levels of recovery often require heart transplantation (Elkayam, 2014; Loyaga-Rendon et al, 2014; Pillarisetti et al, 2014; Sheppard et al, 2014).
Even after complete recovery from PPCM, the risk of recurrence in subsequent pregnancies remains high and left ventricular function can deteriorate again. A left ventricular function ≥ 55% was the most important determinant for freedom from relapse in a post-PPCM pregnancy in a study by Fett et al (2015). A left ventricle diameter >60 mm or ejection fraction <30% is an indicator of poor prognosis (Sliwa et al, 2010).
Subsequent pregnancies, counselling and contraception
It remains difficult to advise patients about future pregnancies, as recurrence is unpredictable. Much of the literature advises against future pregnancies (Lampert and Lang, 1995; Hilfiker-Kleiner et al, 2015), but if a woman is accepting of a recurrence risk and has a subsequent pregnancy, it is important that TTE surveillance of left ventricle function is in place every 4–6 weeks, with an agreement between the woman and cardiologist that if left ventricle function declines pregnancy must be interrupted.
The risk of left ventricle function declining is higher with persistent left ventricle dysfunction before their subsequent pregnancy, whereas if left ventricle function has recovered fully the outcome is more optimistic. However, an uneventful pregnancy cannot be guaranteed, and approximately 20% of women may have a recurrence of PPCM associated with a substantial decrease in left ventricle systolic function (Elkayam et al, 2001; Fett et al, 2010).
Women with PPCM need counselling about contraception. Intrauterine devices are effective and long-lasting forms of contraception that do not increase the risk of thromboembolism. However, oestrogen-based contraception increases the risk of thromboembolism and should be avoided if there is significant left ventricle impairement (ejection fraction <40%) but intramuscular, subcutaneous and subdermal forms of progesterone-only contraception are safe irrespective of left ventricle function. Sterilisation methods can be considered; however, left ventricle function must be taken into account when planning any operative procedure as general anaesthesia may depress left ventricle function, albeit transiently.
The relationship between a pregnant mother and a midwife is unique, and provides an excellent opportunity for midwives to counsel patients about the most appropriate post-discharge contraceptive, by explaining each contraceptive method's efficacy, the risks associated with administration and long-term use and the contraceptive benefits. Because of the psychological impact, women should be counselled carefully and educated about the effective alternatives.
Patients can also be directed to reputable websites and support groups. There are multiple benefits to participation in the online forum including exchange of information, anecdotes and support.
Conclusion
PPCM results in a decline in left ventricle function and onset of heart failure. It may be a mild self-limiting illness or it can lead to severe heart failure and death. It remains a poorly understood, rare disease, which is often misdiagnosed or diagnosed late, resulting in severe complications for mother and baby. An increased awareness of the condition is needed to ensure an early diagnosis is made and treatment is implemented, as this will improve outcomes and save lives.

