Postpartum haemorrhage continues to be the leading cause of maternal mortality and morbidity in the world (Bienstock et al, 2021). Although death from postpartum haemorrhage is considered a preventable event, postpartum haemorrhage is still responsible for around 8% of maternal deaths in developed countries and 20% in developing countries (Bienstock et al, 2021). A primary postpartum haemorrhage is typically understood as blood loss of ≥500ml within 24 hours of birth (World Health Organization (WHO), 2012). However, the blood loss threshold used by clinicians to trigger interventions may be higher or lower, depending on a woman's situation. Healthy pregnant women can endure greater blood loss, while loss of <500ml may cause severe adverse consequences in women with anaemia or chronic disease (Faysal et al, 2023; Glonnegger et al, 2023). Individualisation of care is therefore important not only to understand a woman's underlying chance of having a postpartum haemorrhage, but also the effect that it might have.
Evidence-based tools, such as interdisciplinary guidelines, care bundles, checklists and protocols, have contributed to improving maternal outcomes and reducing maternal mortality (Arora et al, 2016). In a major US programme, the severity of postpartum haemorrhage and the use of blood products significantly declined as a result of the introduction of these tools (Shields et al, 2015). In the UK, the ‘Obstetric Bleeding Strategy Cymru’ protocol has improved outcomes across Wales with its routine gravimetric blood loss assessment and volume-related escalation process (call for help at 500ml, call senior staff and conduct bedside clotting assessment at 1000ml, transfer to theatre and activate massive obstetric haemorrhage policy at 1500ml). It is now widely adopted across the UK, and the subject of a large cluster randomised trial (Bell et al, 2020; 2021). Additionally, these protocols in combination with clinical tools, such as shock index and early warning scores, have a good ability to predict unfavourable outcomes in women with postpartum haemorrhage (Henriquez et al, 2018).
Recent evidence shows that the incidence of postpartum haemorrhage is increasing, particularly in developed nations and high-resource settings (Knight et al, 2009; Huang et al, 2023; Pettersen et al, 2023). Although the reason for this is unclear, the rise in underlying risk factors of obesity, pre-eclampsia and caesarean section are all thought to be important (Ford et al, 2015; Weeks, 2015). Additionally, although numerous risk factors have been determined, postpartum haemorrhage remains unpredictable, and even the lowest risk women may suddenly experience this obstetric emergency (Huang et al, 2023). This means that all pregnant women are considered at risk for postpartum haemorrhage, imposing a heavy burden on healthcare services in terms of managing prevention and treatment.
Is postpartum haemorrhage really ‘unpredictable’?
It is commonly stated that postpartum haemorrhage is unpredictable, and so midwives and obstetricians always need to be prepared (Weeks and Neilson, 2015). While this is true to a certain extent, it is also clear that the risk of postpartum haemorrhage is far higher in a woman with three previous caesarean sections and placenta praevia than it is in a low-risk home birthing parous woman in spontaneous labour (Thams et al, 2022; Huang et al, 2023). Thus, while the chance of postpartum haemorrhage can never be removed, it can be useful to identify higher-risk women to plan their care.
It is also important to note that postpartum haemorrhage is only a symptom and consists of several underlying pathologies. The risk of each pathology is affected to a different degree by various risk factors (Green et al, 2016). For example, a woman with placenta praevia is at risk of surgical blood loss. In contrast, a home birthing parous woman is at very low risk of surgical blood loss, trauma or atonic uterus, but still has a risk of retained placenta, which could unexpectedly result in severe postnatal bleeding (Olsen and Clausen, 2023). For a nulliparous woman with induced labour having a caesarean section at 9cm for dystocia unresponsive to many hours of oxytocin augmentation, the risk is of atonic uterus (Alexander et al, 2023). Therefore, not all risk factors point towards the same type of postpartum haemorrhage.
Risk factors are currently categorised into three groups, including maternal, fetal and pregnancy-related factors (Sade et al, 2022). Through a meta-analysis, Ende et al (2021) summarised the evidence and quantified risk factors for atonic postpartum haemorrhage. They identified 47 potential factors and 15 likely factors, but there were still 32 factors showing conflicting or unclear evidence. This provides a major obstacle to postpartum haemorrhage prediction: a lack of agreement and consensus on the risk factors. Additionally, these factors may be affected by demography and epidemiology, and the evidence supporting or denying each factor can fluctuate (Sade et al, 2022). Therefore, postpartum haemorrhage prediction tools are never going to provide a complete solution to postpartum haemorrhage care, especially given that clinical staff still need to be prepared for postpartum haemorrhage in all women during birth.
Despite these reservations, the obstetric haemorrhage toolkit developed by the California Maternal Quality Care Collaborative is considered the first postpartum haemorrhage predictable tool to assist obstetricians, midwives and healthcare organisations with prompt recognition and response to bleeding (Bingham et al, 2010; Main et al, 2017). In 2017, the Association of Women's Health, Obstetric and Neonatal Nurses (2017) also designed a postpartum haemorrhage risk assessment table to guide clinical decision making. Shortly thereafter, in 2019, the Safe Motherhood Initiative in the USA released the ‘Maternal Safety Bundles for Obstetric Haemorrhage’ (American College of Obstetricians and Gynecologists (ACOG), 2019a). Developed by expert consensus, these three tools provide the probability of postpartum haemorrhage, depending on the presence and absence of its risk factors (Table 1). Although there have been limitations, recent studies demonstrated that the use of these tools has contributed to improved maternal outcomes when compared to traditional risk assessments by individual clinicians (Dilla et al, 2013; Kawakita et al, 2019; Ruppel et al, 2020).
Table 1. Comparison of postpartum haemorrhage predictive tools used in the USA
| Risk level | California Maternal Quality Care Collaborative | Association of Women's Health, Obstetric and Neonatal Nurses | Maternal safety bundle for obstetric haemorrhage |
|---|---|---|---|
| Low | Admission and labour risk factors:
|
|
Admission and labour risk factors: None.Intrapartum:None. |
| Birth and ongoing postpartum risk factors: None. | |||
| Medium | Admission and labour risk factors:
|
|
Admission and labour risk factors:
|
Birth and ongoing postpartum risk factors:
|
|||
| High | Admission and labour risk factors:
|
|
Admission and labour risk factors:
|
Birth and ongoing postpartum risk factors:
|
|||
Intervention:
|
|||
To develop this concept further, there are ongoing attempts to develop an individual-level postpartum haemorrhage risk index, instead of stratifying the risk from low to high. These are being explored through the use of new statistical models, machine learning and artificial intelligence (Rajkomar et al, 2019). Several models have been developed and studied for postpartum haemorrhage prediction, but none have yet been validated and implemented (Neary et al, 2021).
Although identification of postpartum haemorrhage risk factors has received much attention, there are still many women who experience a postpartum haemorrhage without any risk factors (Huang et al, 2023). Thus, every woman needs to be considered at risk for postpartum haemorrhage after giving birth, and at every birth, maternity care staff must be ready and prepared to manage this condition (Abdul-Kadir et al, 2014; Evensen et al, 2017).
Deciding when to treat postpartum haemorrhage
An accurate assessment of blood loss in the third stage of labour is important for two distinct reasons. The first is to make the initial diagnosis of postpartum haemorrhage, and the second is to determine how severe it is and when to escalate treatment (Natrella et al, 2018; Powell et al, 2022). Previous research has often incorrectly considered the two together and assumed that accurate quantification of blood loss volume will solve both problems.
However, most clinicians do not use blood loss volume to initiate postpartum haemorrhage care, relying instead on intuition, ‘hunch’ and a woman's underlying condition (Hancock et al, 2015). Thus, a woman having a second stage caesarean section after a long labour may easily have 800ml blood loss during an uncomplicated caesarean section and not have received any specific postpartum haemorrhage treatment. In contrast, a woman with underlying anaemia and twins who starts to trickle immediately after vaginal birth might be given uterotonics to induce contraction or tonicity of the uterus after the loss of only 100ml, because of a recognition of the underlying risk and the wish to initiate treatment early.
In postpartum haemorrhage simulations using manikins, UK clinicians started uterotonics as soon as they considered the blood loss abnormal, with none awaiting any specific blood loss volume (Hancock et al, 2015). One, however, failed to recognise the mannikin's slow trickle of blood and ‘discharged the women back to the ward’ without further intervention, demonstrating that the initiation of postpartum haemorrhage treatment is far more complex than simple quantification.
Within limits, the exact timing of treatment initiation can be individualised to a woman's underlying risk and cause of postpartum haemorrhage. The most critical element is that it is not missed, either through a neglected slow trickle, or the slow filling of the uterus or vagina with blood. To prevent these, immediate postnatal monitoring of vaginal loss, uterine fundal height and maternal blood pressure and pulse are all critical. It is important not to neglect them simply because vaginal loss appears to have stopped. Quantification of vaginal blood loss at all births is also recommended, not only to ensure that a postpartum haemorrhage of 500ml is not missed, but also so as to triangulate with any postnatal symptoms. Table 2 outlines a proposed protocol aimed at assisting maternity care personnel in standardising the assessment, diagnosis and postpartum haemorrhage treatment. This protocol also encourages individualisation of care for women with potential postpartum haemorrhage risks.
Table 2. Suggested protocol for assessment, diagnosis and initiation of postpartum haemorrhage treatment
| Stage | Action |
|---|---|
| Assessment of blood loss | Change soiled drapes immediately after birth to remove amniotic fluid |
| Measure all subsequent loss until no loss for 15 minutes | |
| Collect blood and weigh all soiled swabs | |
| Monitor for hidden blood loss by palpating top of the uterus (check height, tone and centrality), blood pressure and pulse every 15 minutes in the first hour (or until clinically stable), then hourly for the next 5 hours | |
| Document urine void within 6 hours | |
| Diagnosis and initiation of treatment | Can be individualised (up to 500ml) according to woman's situation and underlying risks |
| Once loss reaches 500ml, treatment must be initiated unless already started or blood loss has stopped | |
| Ensure scribe present to regularly weigh blood-soaked swabs and call out blood loss when it reaches 1000ml, 1500ml, 2000ml, 2500ml, etc | |
| Escalate care, assess clotting and treat according to local protocols as loss increases |
Accurate measurement of blood loss volume is mainly important to manage an ongoing postpartum haemorrhage and escalate multidisciplinary care in a timely manner (Kumaraswami and Butwick, 2022; Forbes et al, 2023). Visual estimation and cumulative, quantitative measurement are two common methods to assess blood loss in clinical practice (Lilley et al, 2015; Natrella et al, 2018; Powell et al, 2022). Visual estimation is quick, simple and free, and allows midwives and doctors to recognise and respond to ongoing bleeding (Natrella et al, 2018). However, estimates are commonly inaccurate, especially at the extremes of blood loss, with a tendency to ‘normalise’ values (Bamberg et al, 2016). While underestimation occurs in situations with higher blood loss, overestimation takes place in cases with lower amount of blood loss (Davis et al, 2019).
To improve the accuracy of assessment, quantitative blood loss has been suggested as an objective approach (Powell et al, 2022). This can be carried out using either the gravimetric method (based on weighing of swabs) or volumetric method (blood loss collection in waterproof drapes or suction bottles). Each can only start after discarding the amniotic fluid expelled with the baby. Although routine measurement of cumulative blood loss can seem complex and laborious to implement in a busy maternity department (Federspiel et al, 2023), quantitative measurement should replace visual estimation of blood loss in all women wherever possible, so as to start timely intervention and prevent ‘missing’ cases of postpartum haemorrhage (ACOG, 2019b; Hire et al, 2020). This is now recommended by all leading authorities around the world, including the WHO (2012), the Royal College of Obstetricians and Gynaecologists (RCOG, 2017), the ACOG (Committee on Practice Bulletins-Obstetrics, 2017), the Society of Obstetricians and Gynaecologists of Canada and the International Federation of Gynsecology and Obstetrics (Escobar et al, 2022; Robinson et al, 2022).
It is common for appropriate postpartum haemorrhage treatment to be delayed because of underestimation of blood loss, and this is a common cause of maternal morbidities and mortalities (Turkoglu and Friedman, 2023). Recent studies show an association between timely intervention and outcomes (Weeks, 2015; Ameh and Althabe, 2022; Turkoglu and Friedman, 2023). Delay in postpartum haemorrhage recognition and treatment results in an increased rate of hypotension, blood transfusion and severe postpartum haemorrhage (Knight, 2019; Federspiel et al, 2022). Conversely, overestimation of blood loss might bring overtreatment, increasing the burden on medical resources and iatrogenic harm (Bláha and Bartošová, 2022).
Prevention and treatment
Once postpartum haemorrhage has been diagnosed, the use of evidence-based care bundles can help healthcare staff to provide interventions in a systematic way (Kumaraswami and Butwick, 2022). These typically consist of a uterotonic, uterine massage, tranexamic acid and intravenous fluids. Postnatal monitoring is also commonly included, using specific cut-offs to trigger escalation as well as prophylactic and therapeutic activities. Results of recent studies conducted in high-resource settings demonstrate that despite the recognised deficiencies, the implementation of these postpartum haemorrhage bundles is safe, effective and associated with declined incidence of postpartum haemorrhage-related morbidity (Shields et al, 2015; Skupski et al, 2017; De Tina et al, 2019; Althabe et al, 2020; Bell et al, 2021).
Uterotonics
Uterotonics are the most common medication used at birth. They are clinically used to induce and augment labour but also to prevent and manage postpartum bleeding. Uterotonics include various categories of agents that cause uterine contraction through different pathways. Detailed understanding of each medication is important and necessary, allowing clinicians to make the right choices for each stage of labour and minimising undesirable effects.
Oxytocin is the first-line choice for prophylaxis and treatment of postpartum haemorrhage and appears in most guidelines across the world. According to the RCOG (2017) recommendations, administration of oxytocin remains the key component of active management of the third stage of labour. Slow intravenous administration of 5IU oxytocin is recommended to prevent postpartum haemorrhage for caesarean birth, in preference to intramuscular injection of 10IU (Soltanifar and Russell, 2012; RCOG, 2017; WHO, 2018).
Heat-stable carbetocin (known as Pabal® in the UK) is a synthesised oxytocin analogue with long-lasting effects and agonist properties. It is also recommended by the WHO (2018) for postpartum haemorrhage prevention as an alternative uterotonic in situations where the cost is comparable (van der Nelson et al, 2017). Carbetocin has also been shown to reduce the need for additional uterotonics when compared to oxytocin at elective caesarean birth (Onwochei et al, 2019), and is recommended for this purposes by the National Institute for Health and Care Excellence (NICE, 2023). For the treatment of postpartum haemorrhage, these measures should be instituted in turn, starting with slow intravenous administration of 10IU oxytocin (WHO, 2012; RCOG, 2017; NICE, 2023) and followed by an infusion of 40IU oxytocin in either 500ml or 1000ml of saline (WHO, 2012; RCOG, 2017; Fouche-Camargo, 2022) to give oxytocin over 4–5 hours until the bleeding is under control.
Ergometrine/methylergometrine is a powerful uterotonic that causes a prolonged tonic uterine contraction and is often combined with oxytocin as Syntometrine® (oxytocin 5IU and ergometrine 500mcg). The recommended dose and route of ergometrine for postpartum haemorrhage treatment is 200–500mcg intramuscularly with a maximum of 1250mcg (Fouche-Camargo, 2022). Ergometrine causes a rapid increase in blood pressure and so should be used with great care in those with hypertension. Typical adverse effects are headaches, hypertension, nausea and vomiting, and women receiving ergometrine should therefore be closely monitored. Many authorities suggest that ergometrine should be reserved for postpartum haemorrhage treatment rather than used for prophylaxis because of its unfavourable adverse effect profile (Gallos et al, 2018; Laganà et al, 2023), but NICE (2023) includes it as a prophylaxis option for high-risk women.
NICE (2023) updated its guidance in September 2023 and included a new table of uterotonics for postpartum haemorrhage treatment. The table varies first-line treatment according to the type of prophylaxis used; in the authors' opinion, this is a rational approach to care, although there is no specific evidence to support or refute it (Table 3).
Table 3. Suggested choice of uterotonic treatment according to type of prophylaxis used in third-stage labour
| Uterotonic used | First-line treatment | Second-line treatment | Additional treatments offered, depending on clinical need |
|---|---|---|---|
| None (physiological management) | Oxytocin plus ergometrine by intramuscular injection (if contraindicated, give carboprost), or oxytocin infusion as soon as intravenous access available | Carboprost intramuscular injection | |
| Oxytocin alone | Ergometrine intramuscular injection (if contraindicated give carboprost), or oxytocin infusion as soon as intravenous access available | Carboprost intramuscular injection | Carboprost intramuscular injection (can be repeated at intervals not <15 minutes up to maximum 8 doses), or misoprostol 800mcg sublingually or rectally (may be used earlier if intravenous route not available), or carbetocin slow intravenous injection |
| Oxytocin plus ergometrine | Carboprost intramuscular injection, or oxytocin infusion as soon as intravenous access available | Repeat carboprost after 15 minutes | |
| Carbetocin | Ergometrine intramuscular injection | Carboprost intramuscular injection | Carboprost intramuscular injection (can be repeated at intervals not <15 minutes up to maximum 8 doses), or misoprostol 800mcg sublingually or rectally |
Prostaglandins used in obstetric practice include PGE1 (misoprostol), PGE2α (carboprost) and PGE2 (dinoprostone). Following oxytocin, they are frequently administered as a second-line treatment, especially if ergometrine is contraindicated. Common side effects of all prostaglandins are fever, shivering, diarrhoea, nausea and vomiting. Globally, the use of misoprostol in postpartum haemorrhage prophylaxis and treatment has been the subject of much attention and research because of its low cost and that is does need refrigeration. The recommended dose for prophylaxis 400–600µg orally, and 800µg sublingually or rectally for treatment (WHO, 2012; 2020; RCOG, 2017; NICE, 2023). High fever and shivering are two most common side effects of misoprostol, but both rapidly respond to paracetamol (Durocher et al, 2010).
The use of dinoprostone is not generally considered for postpartum haemorrhage, although a clinical trial in 2010 found that it is as effective as oxytocin in the prevention of postpartum haemorrhage (Ozalp et al, 2010). However, stronger evidence is required to support the effectiveness of dinoprostone in postpartum haemorrhage prevention and treatment.
Although carboprost (Hemabate®, 0.25mg intramuscularly) is approved for the treatment of postpartum haemorrhage, it is not recommended for prevention (WHO, 2018). A large ongoing trial is comparing its first-line use for postpartum haemorrhage treatment to oxytocin, which will inform its role in postpartum haemorrhage treatment. As a result of the risk of acute bronchospasm, carboprost should not be provided to women who have a history of asthma or substantial pulmonary disease (Gallos et al, 2018; Laganà et al, 2023).
The role of tranexamic acid
In recent years, tranexamic acid, an anti-fibrinolytic agent, began to be used in the treatment of acute bleeding in numerous situations because of its low cost, ease of administration and low incidence of side effects (Sentilhes et al, 2018; Saccone et al, 2020). It has now entered NICE (2023) guidance for use as a treatment for all women with postpartum haemorrhage. Tranexamic acid is a successful intervention for reducing bleeding in pregnant women with postpartum haemorrhage and, from a clinical perspective, has the advantage of doing so regardless of the underlying cause (Shakur et al, 2017). Additionally, recent evidence illustrates that tranexamic acid can be used for both the prevention and treatment of postpartum haemorrhage (Lee et al, 2023; Gedeno Gelebo et al, 2024).
A 2015 Cochrane review on the role of tranexamic acid in postpartum haemorrhage prophylaxis showed that in addition to uterotonics, tranexamic acid can reduce postpartum blood loss, prevent postpartum haemorrhage and reduce the need for blood transfusions after both vaginal birth and caesarean section in women who are at low risk of postpartum haemorrhage (Novikova et al, 2015). Results from a systematic review with meta-analysis of randomised clinical trials conducted in 2022 also demonstrated the effectiveness of prophylactic tranexamic acid in reducing postpartum bleeding (Assis et al, 2023). While there were concerns that universal tranexamic acid prophylaxis could increase the rate of thromboembolism, this side effect appears to be extremely rare (McQuilten et al, 2024). Authors emphasise the importance of further studies to accurately assess side effects and determine the optimal dose to achieve a therapeutic effect with the fewest side effects (Gedeno Gelebo et al, 2024).
Recent studies and systematic reviews have demonstrated that as well as being suitable for prophylactic use, tranexamic acid is a successful treatment for postpartum haemorrhage (Sentilhes et al, 2018; Shakur et al, 2018; Gedeno Gelebo et al, 2024). A Cochrane review demonstrated that the use of tranexamic acid in postpartum haemorrhage treatment contributed to a reduced rate of death from bleeding and laparotomy to control bleeding, without any sign of increase in thromboembolic risk (Shakur et al, 2018). Data from the WOMAN trial suggest that tranexamic acid is most effective when given early after diagnosis. Surprisingly, despite reducing laparotomy and death, there was no effect on rates of hysterectomy, serious maternal morbidity or blood transfusion (Shakur et al, 2017).
A review examining the cost-effectiveness of tranexamic acid concluded that in situations where postpartum haemorrhage and postpartum haemorrhage-related morbidity are common and tranexamic acid is inexpensive, it is likely to be cost-effective (Aziz et al, 2021). One obstacle to women receiving tranexamic acid treatment is the requirement for intravenous injection. This can be a barrier for women giving birth in places where it is difficult to administer an injection. Alternative administration methods would make this life-saving medication available to more women (Ameh and Althabe, 2022). Shakur-Still et al (2022) suggested that, based on the tranexamic acid pharmacokinetic profile, intramuscular administration should be preferable to intravenous injection because of the ease of administration and speed, given the requirement for slow intravenous injection over 10 minutes.
Using the thromboelastogram to guide haematological management
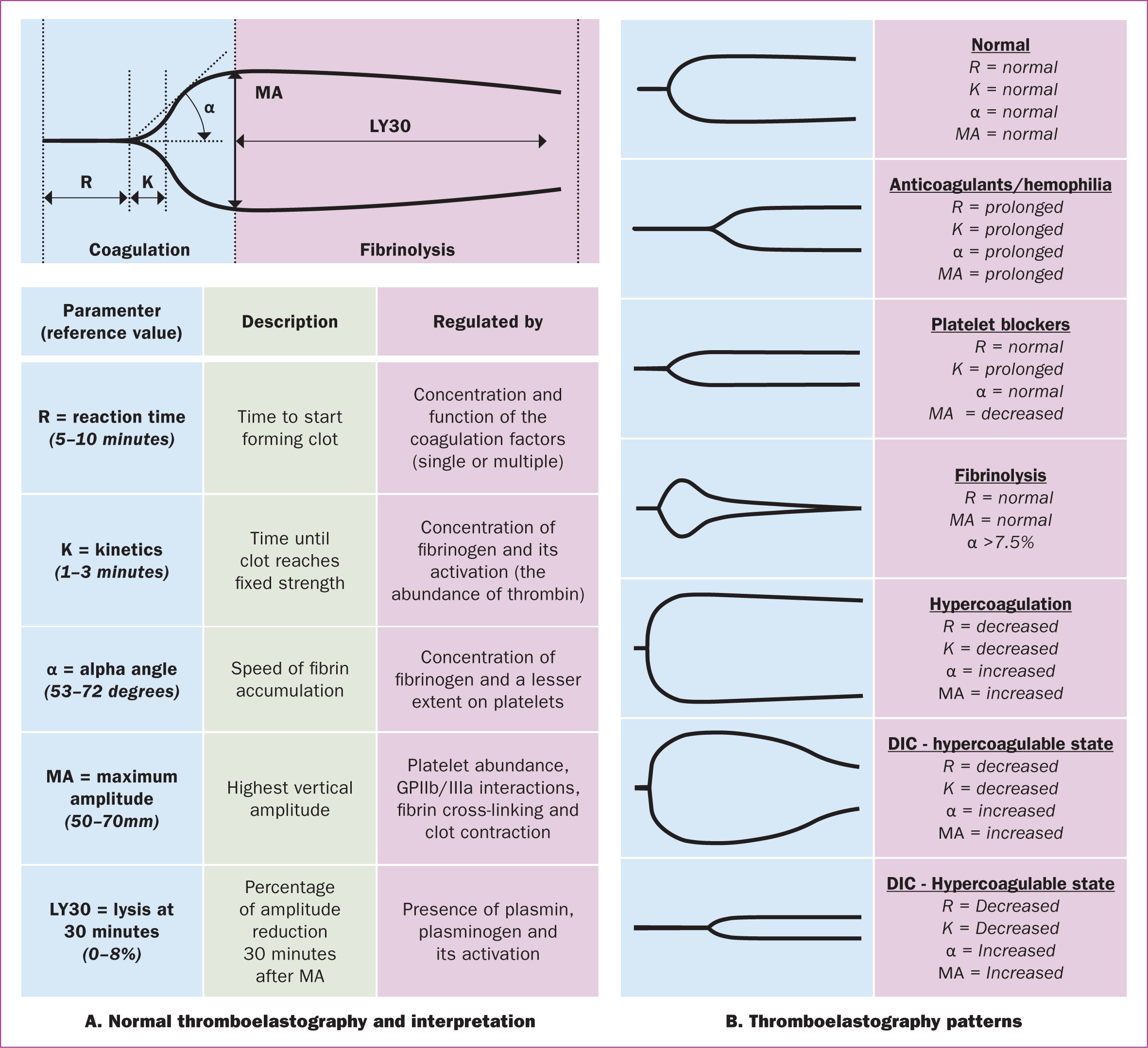
Thromboelastography was developed nearly 70 years ago by Hellmut Hartert, a German doctor, but was not implemented into clinical practice until 25 years later. The initial purpose of this viscoelastic dynamic global test was to track alterations in coagulation and fibrinolysis related to liver transplantation. Thromboelastography is now implemented as a point-of-care device, with applications in various therapeutic domains, thanks to major improvements brought about by technological advancements over the past few decades (Othman and Kaur, 2017).
For decades, the length of time taken for fresh blood to clot in a test tube has been used as a simple bedside test for disseminated intravascular coagulation. Thromboelastography is a development of that same technique. It works by placing a small needle in a vibrating vial of fresh blood. Initially, the needle moves little as the blood is completely liquid. However, as the clot forms, so the needle starts to move with the vial of blood, which shows on a graphic reader. The start of the deflection on the graph shows the initiation of clot formation, and the widest part relates to the maximum strength of the clot before it begins to break down. The shape of the graph can be measured to calculate various aspects of the clotting cascade (Othman and Kaur, 2017).
Thromboelastography has been used in maternity care for early diagnosis and treatment of coagulation disorders in postpartum haemorrhage, and has yielded promising results (Karlsson et al, 2014; Hartmann et al, 2018; McNamara and Mallaiah, 2019; Rigouzzo et al, 2020; Zhu et al, 2022; Khanna et al, 2023). Results of one study showed that when compared to traditional laboratory testing, thromboelastography provides rapid and clinically important information about haemostatic changes associated with massive postpartum haemorrhage, revealing indications for specific blood product therapies at an earlier stage (Karlsson et al, 2014). Thromboelastography also provides a reliable detection of coagulation disorders (hypofibrinogenemia ≤2g/L and/or thrombocytopenia ≤80 000/mm3), assisting clinicians in the rapid diagnosis and treatment of clotting disorders during the progression of postpartum haemorrhage (Rigouzzo et al, 2020). Thus, thromboelastography has a good diagnostic value for peripartum haemorrhage, playing an important role in postpartum haemorrhage prediction and providing clinical evidence for prevention, evaluation and treatment (Perelman et al, 2021; Zhu et al, 2022). It is now in widespread use in the UK as part of the Obstetric Bleeding Strategy Cymru protocol (Bell et al, 2020; 2021). Figure 1 provides a guide to its interpretation (MacIvor et al, 2012).
Future research strategies in prevention and management
Recent studies have shown that postpartum haemorrhage prediction tools have an important role to play in the improvement of maternal outcomes, and many of them have been validated and implemented across the world. However, the use of prediction tools based on available risk factors still need to be used with caution, given that the risk factors have changed over time and that postpartum haemorrhage still occurs in women with almost no risk factors. Instead of stratifying the postpartum haemorrhage risk from low to high prior to labour as at present, a specific ‘postpartum haemorrhage risk index’ may provide a more nuanced and individualised approach to adjusting the postpartum haemorrhage prevention and management strategy. This should also include risks specific to the setting (including low-income countries) and intrapartum risk factors (prolonged labour, caesarean section or instrumental birth). This would improve postpartum haemorrhage risk stratification, early and targeted interventions, patient-centred care, research and quality improvement. The development and validation of a continuous numerical postpartum haemorrhage risk index should be developed, and studies carried out to optimise its accuracy and clinical utility.
Treatment bundles have shown their effectiveness in the management of postpartum haemorrhage, significantly contributing to the reduced rate of haemorrhage-related mortality and morbidity. However, their application has some limitations. Current cut-offs used to trigger prevention and treatment actions seem to be effective in lower risk women but do not represent all groups of risk women. A pregnant woman with anaemia or an underlying heart condition should, of course, receive interventions earlier than those with normal haemoglobin. Therefore, it is necessary to have various cut-offs for initiating treatment, depending on women's underlying health status as well as their level of postpartum haemorrhage risk.
Additionally, there are several barriers that may interfere with bundle application. In low-income settings, where maternity units have a high throughput of births but are less well equipped, the implementation of bundles for all low-risk women would create a huge workload for healthcare staff. As a result, thresholds for care in protocols or bundles should be further researched so that clinicians can individualise care, adjusting them to match the underlying risk of bleeding in the women as well as the resources available in the unit.
Conclusions
Despite massive investments in maternal health services worldwide, postpartum haemorrhage continues to remain a leading cause of maternal death. The use of uterotonics and tranexamic acid are being gradually optimised, helping to improve postpartum haemorrhage outcomes. The development of prediction tools and bundles of prophylaxis and treatment have contributed to the reduced incidence of postpartum haemorrhage-related mortality and morbidity. More research is now required to address the limitations of predictable tools and improve the effectiveness of protocols or bundles used in daily practice in various settings.
Key points
- Evidence-based tools and guidelines have shown promise in improving maternal outcomes, but the lack of consensus on postpartum haemorrhage risk factors hinders the development of accurate prediction models.
- Accurate ongoing quantification of blood loss during the third stage of labour using objective measurement methods is crucial for timely diagnosis and appropriate management of postpartum haemorrhage.
- Uterotonics and tranexamic acid have demonstrated effectiveness in postpartum haemorrhage prevention and treatment, although further research is needed to optimise their use and mitigate potential side effects.
- Thromboelastography is a bedside dynamic test that provides rapid, clinically important information about changes in maternal clotting associated with postpartum haemorrhage, enabling early diagnosis and treatment of coagulation disorders.
- Ongoing research efforts, including the development of an individual-level postpartum haemorrhage risk index and the use of advanced statistical models and artificial intelligence, hold promise in improving prediction accuracy and individualised care for pregnant women at risk of postpartum haemorrhage.
CPD reflective questions
- In your clinical practice, what triggers you to start treatment for postpartum haemorrhage and why? Why are postpartum haemorrhages sometimes missed, and what can be done to overcome this?
- How would the development of an individual-level postpartum haemorrhage risk index help to guide individualised care for pregnant women? What changes in care would you advise to a mother if she scored as high risk?
- What are the main challenges in implementing accurate quantification methods for blood loss, and how can these challenges be addressed to ensure timely postpartum haemorrhage diagnosis and appropriate management?
- What postpartum haemorrhage prophylaxis do you recommend for women in your care and why?
- What are the barriers to implementing thromboelastogram testing in clinical practice, and how can they be overcome to improve postpartum haemorrhage management outcomes?


