From the moment of birth, around 90% of neonates initiate spontaneous respiration within 30 seconds (Resuscitation Council UK, 2016). For approximately 10% of neonates, this process takes a little longer and a degree of support is needed to achieve cardiopulmonary stability (Ersdal and Singhal, 2013). Current guidelines advise clamping and cutting the umbilical cord when resuscitation is required (Resuscitation Council UK, 2016), and the neonate is swiftly removed from the mother's bedside to the resuscitaire for assessment and stabilisation (Yoxall et al, 2015). The anticipation of positive pressure ventilation is the primary reason for this decision; however, this is only required in 3% of cases (Resuscitation Council UK, 2016). Consequently, neonates are denied the benefits of delayed cord clamping (DCC) (Hutchon and Burleigh, 2013), when requiring nothing more than stabilisation which can be provided at the mother's bedside. While the potential harm of immediate cord clamping (ICC) is widely recognised (World Health Organization [WHO], 2012; Hooper et al, 2015; Leslie, 2015; Royal College of Obstetricians and Gynaecologists [RCOG], 2015), this risk is imposed on compromised neonates (Bhatt et al, 2013), those it could be argued would benefit from DCC more than uncompromised neonates.
The optimal management for neonatal resuscitation is driven by formalised guidelines provided by the International Liaison Committee on Resuscitation (ICLOR) (Perlman et al, 2012), using a structured algorithm (Figure 1), and periodic assessment (30 seconds) of heart rate, breathing effort and tone until cardiopulmonary stability is achieved (Resuscitation Council UK, 2016). However, due to the infrequency of extensive neonatal resuscitation, there is a lack of research to advise the optimal time to clamp the cord for compromised neonates (WHO, 2012; Ersdal et al, 2016). Guidelines are predominantly based on data extrapolated from animal studies and those of preterm neonates with subsequent organ immaturity, which does not represent the term neonate's physiological transition to extrauterine life (Vali et al, 2015). Conversely, adverse effects from suboptimal resuscitation are increasingly being noted (Kapadia and Wyckoff, 2013; Saugstad et al, 2014).

Midwives have a professional duty to provide safe, evidence-based care in the best interests of mother and baby (Nursing and Midwifery Council, 2015), and by advocating the simple act of maintaining an intact cord may improve the outcomes for many neonates. DCC lengthens the time to placental separation and confers benefits primarily concerning the net transfusion of blood from the placenta to the neonate (placental transfusion) (Niermeyer and Velaphi, 2013). The fetal-placental circulation contains 110–115 ml/kg of blood (Kluckow and Hooper, 2015). Approximately two-thirds of which circulates the fetus, with one-third remaining in the placenta at any one time to facilitate gas exchange (Mercer and Erickson-Owens, 2014). Blood is transported from the placenta to the fetus through the umbilical cord (via one umbilical vein) and returns back to the placenta (via two umbilical arteries) (Uwins and Hutchon, 2014). Following birth, placental transfusion continues for several minutes to deliver oxygenated blood through the patent umbilical vein (Niermeyer and Velaphi, 2013). When clamping of the cord is delayed, the neonate has access to approximately 20–30% increased net placental blood volume (Mercer and Erickson-Owens, 2012). In a key study, Yao et al (1969) demonstrated that term neonates with ICC had 70 ml/kg of blood compared to 90 ml/kg in those with DCC.
Whilst clamping and cutting of the umbilical cord undoubtedly plays an integral role in the neonate's transition to extrauterine life, the rationale remains controversial (Lawton et al, 2015). Advocates for DCC insist that the umbilical cord should be left until pulsation has ceased (Burleigh and Tizzard, 2015), as placental transfusion is heavily influenced by physiology, and therefore varies between each neonate (Kluckow and Hooper, 2015; RCOG 2015). Such advocates extend the benefits of DCC to compromised neonates, stating that resuscitation with an intact cord should be provided wherever possible (Burleigh and Tizzard, 2015; Salem, 2017).
Emerging research suggests that cord clamping should not occur before the onset of respiration (Bhatt et al, 2013) as this results in a more stable physiological transition for the compromised neonate who is at greater risk of hypoxic insult during this time (Hooper et al, 2015). However, to date, this is not acknowledged in current neonatal resuscitation guidelines, therefore timing relies heavily on the decision of the healthcare professional (Chalkais et al, 2013). The WHO (2012) states that resuscitation with the cord intact may be provided by an experienced practitioner; a common practice noted in low-resource settings, but yet to reach the hospital environment. As a result, in a medicalised environment, when the need for resuscitation is anticipated, resuscitative intervention takes precedence, which usually means clamping and cutting the cord.
What are the benefits of delayed cord clamping?
A delay in clamping of the umbilical cord provides a gentle, physiological transition to extrauterine life with benefits such as increased blood pressure and perfusion of vital organs, and higher peripheral temperature and breastfeeding rates (Mercer and Ericksson-Owens, 2012).
More notably, DCC is widely advocated to prevent iron deficiency (anaemia), further associated with impaired neurodevelopment and increased child mortality (WHO, 2014). DCC provides a 50% increase in red cell volume, enough to meet the increasing demands of iron up to the age of 6 months (Mercer and Erickson-Owens, 2014). This is of particular relevance as it is during this time when rapid neurological development occurs (Cusick et al, 2018). McDonald et al (2013) studied the outcomes of 3 911 mother and neonate pairs and found that DCC for at least one minute significantly increased birthweight and serum haemoglobin levels; improvements in iron stores persisted to 3–6 months. Conversely, neonates who received ICC were twice as likely to become iron-deficient when compared to outcomes of DCC. Similarly, Andersson et al (2011) found a lower incidence of iron deficiency at 4 months of age. In a bid to determine any long-term benefits, a follow-up trial was conducted, which included 69% of the original participants, where better fine motor skills were found in the DCC group compared to those who received ICC (Andersson et al, 2015). It could be considered that DCC not only prevents iron deficiency in the neonate but may also offer neuroprotection.
More recently, research shows DCC offers lifelong benefit through transplantation of hematopoietic stem cells (HSCs) which would otherwise be lost in ICC (Mercer and Ericksson-Owens, 2012; Lawton et al, 2015). It is postulated that HSCs offer considerable therapeutic benefit which has been successfully used to treat disorders such as: cerebral palsy, type 1 diabetes, autism and asthma (Tolosa et al, 2010; Gruneberg and Crozier, 2015; Sun et al, 2016). As the aetiology of neonatal disease often refers to organ immaturity (Mercer and Ericksson-Owens, 2012), Tolosa et al (2010) argue that the loss of stem cells at birth through ICC may predispose neonates to chronic disease. More recently, preliminary trials suggest that HSCs have the potential to aid recovery from hypoxic-ischemic encephalopathy (Cotton et al, 2014). These findings give rise to a debate on whether stem cells play an intrinsic role to repair intrapartum injury (Uwins and Hutchon, 2014), thus preventing the onset of disease which may prevail through later life. More research to analyse the long-term outcomes of ICC compared with DCC would help in further understanding this matter.
What are the perceived risks of DCC?
Primarily, barriers which prevent the implementation of DCC in clinical practice derive from concerns that polycythemia, respiratory distress and hyperbilirubinemia (jaundice) may be caused as a result of ‘overtransfused’ blood (Mercer et al, 2017). Following placental transfusion, there is a marked increase in haematocrit; largely influenced by the time allowed before the umbilical cord is clamped (Mercer et al, 2017). Neonatal polycythemia is defined as a venous haematocrit >65%, further associated with hyperviscosity, organ dysfunction and neurological sequelae (Sarkar and Rosenkrantz, 2008). Saigal and Usher (1977) first coined the phrase ‘symptomatic neonatal plethora’ to describe symptomatic neonates with raised haematocrit following DCC.
However, their analysis failed to consider factors, such as: cigarette smoking, maternal hypertension or intrauterine growth restriction, which may cause erythropoiesis initiated by a hypoxic intrauterine environment and raise the haematocrit (Mercer, 2001; Sarkar and Rosenkrantz, 2008; Teramo and Widness, 2009). Therefore, polycythemia may have been induced by pre-existing pathology rather than placental transfusion. Additionally, symptoms were more ‘generalised’ and could be attributed to underlying conditions (Saigal and Usher, 1977). In a systematic review, Hutton and Hassan (2007) identified an increased risk in neonates who received DCC however, statistical significance was lost when only high-quality data was considered. Furthermore, no data published from 1980 onwards supports a link between ‘symptomatic’ polycythemia and DCC (Mercer and Erickson-Owens, 2012; McDonald et al, 2013; Fogarthy et al, 2018), therefore these claims appear unproven.
Neonatal hyperviscosity, secondary to polycythemia, is thought to delay the transudation of lung fluid which has led to concerns of respiratory distress at birth. This was first raised by Oh et al (1966) who found a higher respiratory rate in late clamped neonates. They state that DCC caused pulmonary oedema, which produced more rapid, shallow breathing (Oh et al, 1967). Yao et al (1971) identified increased expiratory grunting following DCC, but this appeared to resolve within four hours of birth. A noticeable rise in viscosity has been reported in the study of term neonates following DCC (Linderkamp et al, 1992). More interestingly, a simultaneous decrease in vascular resistance and increased pulmonary vasodilation was also noted, demonstrating the neonate's physiology supports a normal transition and corrects viscosity. Katheria et al (2017a) suggest that DCC stimulates an ‘erectile’ response, which results in an increase of pulmonary arterial pressure, thus conveying a protective benefit for the neonate. In their meta-analysis, Hutton and Hassan (2007) did not identify any significant difference in the risk of tachypnoea or respiratory grunting following DCC, nor was any increase in admission to neonatal care noted. A similar finding was also reported in the Cochrane review (McDonald et al, 2013). From this, it would appear that hyperviscosity is an adaptive mechanism which facilitates extrauterine transition and confers no demonstrable adverse effects.
Similar results can be found in research on the incidence of hyperbilirubinemia following DCC. In utero, fetal haemoglobin has a higher affinity for oxygen to compensate for the hypoxic environment. Following birth, the lungs take over and air breathing increases oxygen levels; excess red bloods cells are broken down where bilirubin becomes the by-product (Pincombe et al, 2015). The neonate's immature liver is unable to process circulating levels of bilirubin which may result in physiological jaundice, a common occurrence in approximately 60–80% of neonates (National Institute for Health Care Excellence, 2014). While significantly elevated levels of serum bilirubin may cause harm, it is suggested that those within normal range offer antioxidant properties which protect neurodevelopment (Zahir et al, 2015). Therefore, a higher level may offer more protection as long as it remains in normal range.
The effect of cord clamping on the neonate's adaptation to extrauterine life
In utero, effectively, the placenta and umbilical cord ‘breathe’ for the neonate (Morley, 2005). The lungs are liquid-filled and do not have a purpose for oxygen absorption; gas exchange occurs across the placenta. Oxygenated blood from the placenta is circulated via three vascular shunts: the ductus venosus, the ductus arteriosus and the foramen ovale (Figure 2).
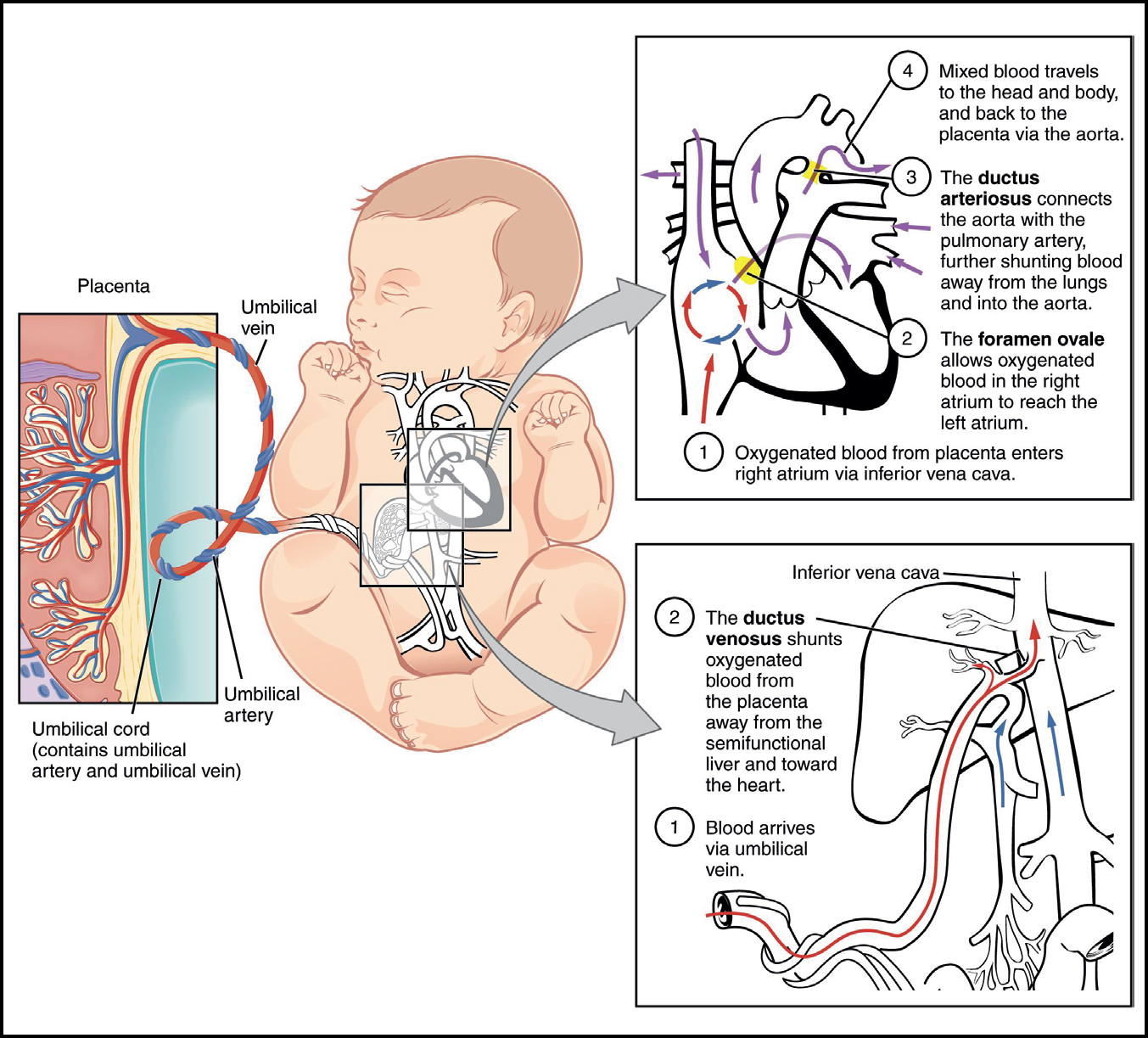
The uninflated lungs maintain high pulmonary vascular resistance (PVR) (Hooper et al, 2015). Pulmonary blood flow (PBF) is low (8%) (Hooper et al, 2015); right ventricular output (preload) predominantly evades the lungs via the ductus arteriosus where it either flows into systemic circulation or returns to the placenta via the umbilical arteries (Polglase and Hooper, 2006). As a result, pulmonary venous return is low and left ventricular preload is dependent on umbilical venous return derived from the placenta via the ductus venosus and foramen ovale (Bhatt et al, 2013; Kluckow and Hooper, 2015). The majority of healthy term neonates spontaneously breathe before the umbilical cord is clamped (Resuscitation Council UK, 2016). Pulmonary ventilation triggers a significant decrease in PVR and subsequent 8-to 10-fold increase in PBF (50%) as right ventricular output flows to the lungs (Rudolph, 1977; Niermeyer, 2015).
Pulmonary venous return sequentially increases to the left atrium whereby closure of the foramen ovale occurs soon after (Riviere et al, 2017). Moreover, ventilation of the lungs increases partial pressure of oxygen in arterial blood (PaO2); this stimulates constriction and closure of the ductus arteriosus and umbilical arteries (Hutchon, 2015). Umbilical venous return continues, thereby supporting pulmonary vasculature without compromising other vital organs (Hutchon, 2008; Graves and Haley, 2013). Reassurance of pulmonary ventilation is normally assumed following a vigorous cry at birth. However, it is an adequate blood volume, not oxygen supply which is vital to facilitate the neonate's physiological transition (Mercer et al, 2009). Thus, indicating that the umbilical cord has a vital role to play in the transition to extrauterine life.
The blood volume theory depicted by Mercer and Skovgaard (2002) highlights the value of maintaining placental circulation to support optimal neonatal transition at birth (Figure 3). This challenges current practice where ventilation is prioritised over blood volume through the practice of ICC. When the cord is clamped before ventilation occurs, umbilical venous return is markedly reduced, followed by a rapid increase in systemic vascular resistance (Hooper et al, 2015). Consequently, arterial pressure increases rapidly by approximately 30%, triggering a similar increase in cerebral blood flow (Mercer and Erickson-Owens, 2014). As cardiac output is also decreased, the latter increase is transient and rapidly decreases until the onset of ventilation, when circulation reaches an equilibrium.
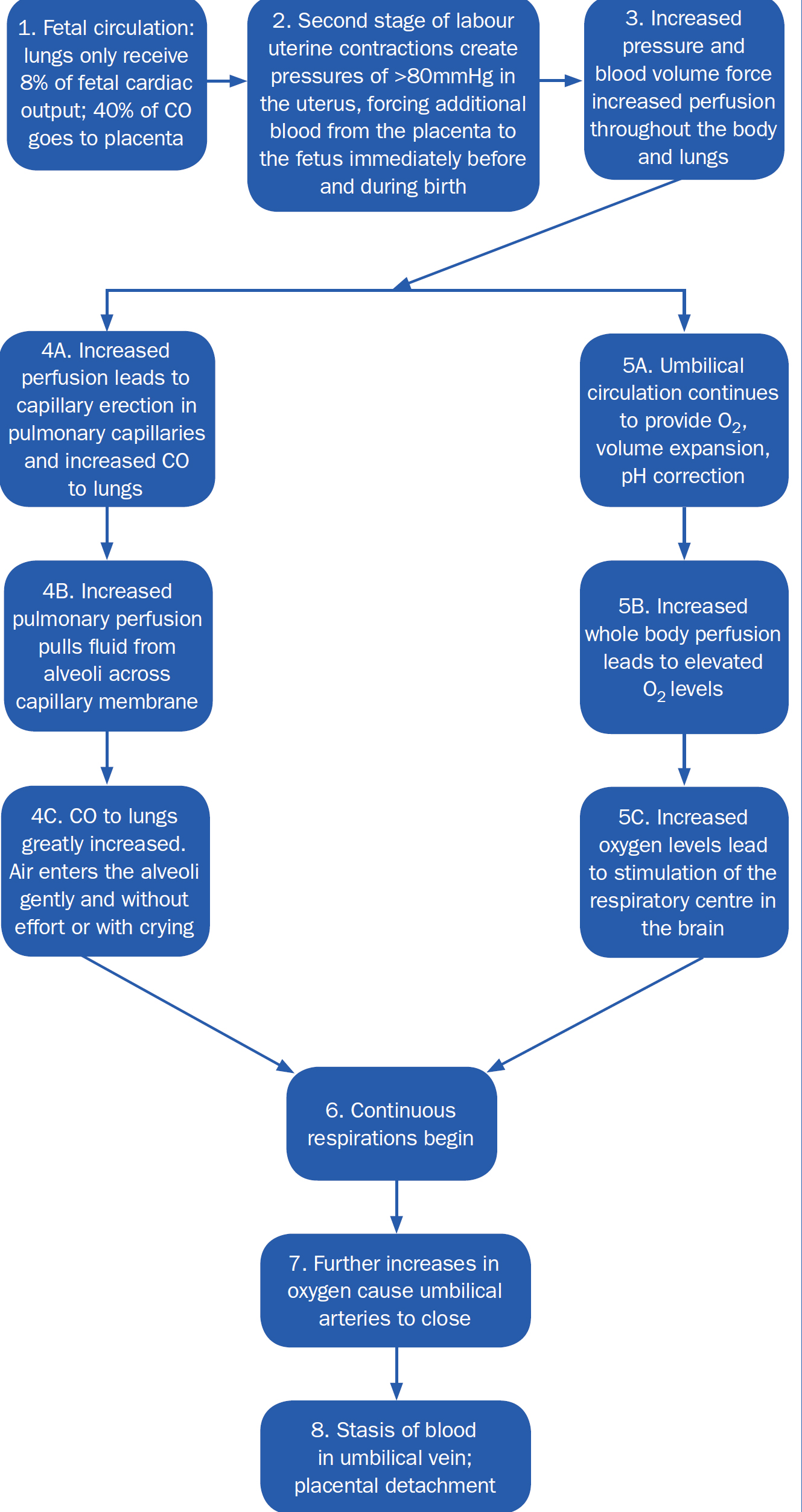
These noted large swings in cardiac output affect the stability of circulatory transition and increases the risk of cerebral insult (Hooper et al, 2015). In a study of preterm lambs, Bhatt et al (2013) showed a rapid decrease in heart rate (bradycardia) and right ventricular output, along with increased arterial pressure following ICC. Those lambs ventilated before the cord was clamped maintained cardiovascular stability during neonatal transition, representing a higher circulating blood volume (Mercer and Erickson-Owens, 2014). Their findings challenge the current rationale for cord clamping which does not consider physiology in arbitrary times, suggesting pulmonary ventilation should precede cord clamping to promote a smooth transition in venous return from umbilical to pulmonary circulation (Bhatt et al, 2013). Therefore, a delay in cord clamping, which preserves blood volume, would be more beneficial than ICC.
Is ICC an iatrogenic cause of resuscitation?
ICC may predispose the need for resuscitation, especially for those neonates with ineffective pulmonary ventilation (Raju, 2013). Following ICC, umbilical venous return ceases and left ventricular output is determined by pulmonary blood flow (Bhatt et al, 2013). Thus, any significant delay between clamping the cord and aeration of the lungs imposes hypoxia on the neonate, which could exacerbate ischemic insult (Martinello et al, 2017). In a computer simulation to explore transitional circulation, Yigit et al (2015) demonstrated DCC preserved arterial oxygenation when pulmonary ventilation was delayed, which supports the existing in vivo data (Sommers et al, 2012; Bhatt et al, 2013).
The degree of harm caused by ICC varies widely; some disorders are self-limiting however, significant hypoxia can lead to lifelong neurological sequelae (Hutchon, 2015). If the cord is still pulsating, DCC maintains an adequate blood volume which prevents ischemic insult (Katheria et al, 2017c). Despite this, compromising the neonate's only life support system remains standard care. ICC deprives the neonate of approximately 30% total blood volume which resides in the placenta. When considering adults develop hypovolemic shock from a blood loss of 15% (Derosby, 2015), in effect, the immediately clamped neonate has undoubtedly endured a significant haemorrhage (Evans, 2012). The healthy term neonate may establish a normal transfusion of blood before the clamp is applied. However, a compromised neonate is often born hypotonic, pale and shows little respiratory effort; typical symptoms of hypovolemia (Mercer and Erickson-Owens, 2014). Therefore, when ICC is performed in order to initiate resuscitation, the blood loss is greater and prevents perfusion of vital organs.
Instances of blood loss significant enough to cause hypovolemia are seen in cases of cord compression, such as in shoulder dystocia, where the need for DCC is vital to prevent cardiac asystole (Mercer et al, 2009; Uwins and Hutchon, 2014). Cord compression leads to occlusion of the umbilical vein and subsequent loss of oxygenated blood which supplies the fetus; when this persists, compression of the body during birth encourages a transfer of blood from the peripheral to central circulation which maintains a steady heart rate and blood pressure (Mercer and Erickson-Owens, 2014). Following birth, this compression is released, and blood is rapidly transferred back to the peripheral circulation (Uwins and Hutchon, 2014), causing a loss of central perfusion; the neonate's heart slows, resulting in hypovolemic shock (Mercer et al, 2009). Routine management is to immediately clamp and cut the cord, followed by ventilation of the lungs. Ironically, it is an increase in blood volume which is needed, not oxygen (Hutchon, 2016); the lack of resilience in the neonate is due to the practice of ICC.
What is the minimum neonatal heart rate before hypoxia ensues?
The heart rate is considered to be an important determinant of wellbeing at birth. A heart rate that remains low, that is, below 100 beats per minute, is often taken as an indicator of hypoxic insult (Sharma, 2017). This usually warrants active resuscitation, and normally the first action in order to commence this would be to immediately cut the cord (Hutchon, 2015). However, whether this is necessary is debatable, as discussed throughout this article.
The heart rate of a healthy neonate may be ‘physiologically’ lower than normal values. Dawson et al (2009) studied the heart rates of 468 preterm and term neonates in the first minutes following birth. They found heart rates were reduced immediately after birth, yet Apgar scores at one minute did not indicate hypoxia. Smit et al (2014) corroborates this, but also notes, more interestingly, that neonates in this study were also placed in immediate skin to skin following birth, a factor associated with lower cortisol levels and reduced response to stress (Takahashi et al, 2011; Smit et al, 2014). All neonates recovered in the absence of hypoxia, demonstrating other physiological factors may be the cause of bradycardia (Hillman et al, 2012). However, bradycardia in the first minute may lead a midwife to ICC to initiate resuscitation. As a result, the cardiovascular consequences for an already low heart rate would be exacerbated (Bhatt et al, 2014).
Does DCC prevent the need for resuscitation at birth?
In a retrospective study, Brookes et al (2013) analysed the effect of DCC for term neonates following implementation of DCC in official guidelines. Remarkably, they noted a significant difference in the rate of active resuscitation, which decreased from 15% to 4.08%. Furthermore, the rate of admission to special care for respiratory support also decreased from 4.5% to 2.55%. They concluded that with an intact cord, neonates responded more readily at birth, reducing the incidence of unnecessary resuscitation (Brookes et al, 2013). The significance of these results led to the introduction of bedside resuscitation, more recently supported with the LifeStart trolley at Northumbria Specialist Emergency Care Hospital in Cramlington (Salem, 2017). However, as no further research has been disseminated, similar trusts may be less likely to take on such a change.
There appears to be a dearth of research concerning the breathing status or methods of resuscitation used on term neonates which compare ICC with DCC (Brookes et al, 2013; Katheria et al, 2017b). Kaempf et al (2012) conducted a ‘before-after’ study, which compared DCC (defined as 45 seconds) with ICC in relation to provision of resuscitation at birth and found the incidence of resuscitation was lower in the DCC group. Group selection was limited to preterm neonates who did not require major resuscitation, however the results were still encouraging. If DCC is tolerated by preterm neonates with an immature respiratory system, without demonstrable adverse effects, this provides vital reassurance of the efficacy for term neonates.
In their 2012 guidelines, the WHO advocate a more gentle approach for neonates who do not initiate respiration at birth, through tactile stimulation of the back before clamping the cord and initiating positive pressure ventilation (PPV). In the absence of published research, the guidelines were based in collaboration with the Guidelines Development Group (GDG) (Ersdal et al, 2016), with key members who have successfully developed the Helping Babies Breathe (HBB) protocol, which advocates DCC in resource-limited areas using a bag and mask (Hutchon and Bettles, 2016). The key principle places immediate emphasis on drying and stimulation to breathe within the first ‘golden’ minute before the cord is clamped (Bang et al, 2016). Implementation of the protocol has been shown to reduce neonatal mortality in the first 24 hours of birth and prevent overuse of resuscitative methods in low-resource settings (Singhal et al, 2012). Such a simplistic approach may provide the obvious solution to enable resuscitation to take place without severing the umbilical cord.
In low-technology settings, such as home birth, there is no need to ‘cut and run’ to the resuscitaire (Gruneberg and Crozier, 2015). The essential requirement is warmth; a flat surface and ventilation support with a bag and mask can be provided with the cord intact as the neonate lies next to the mother (van Rheenan, 2007; Hutchon and Beetles, 2016). It must also be remembered that neonates are essentially born with their own resuscitation equipment and while the umbilical cord remains intact, oxygenated blood continues to be delivered for at least 90 seconds (Reed, 2016). Anecdotal research suggests midwives were early adopters of DCC before the physiological benefit was highlighted (Hutton et al, 2016), this could be attributed to reflection on their practice and intuition of benefits gained from DCC. It could be considered that their desire to protect the mother-infant dyad provides an alternate view of best practice for cord clamping. Therefore, it is of no surprise that evidence has emerged which describes midwives instinctively supporting resuscitation with the cord intact (Fulton et al, 2016). Although this descriptive study was relatively small, it demonstrates midwives advocating the benefits of DCC for the neonate. Interestingly, in the hospital, only 12% performed resuscitation with the cord intact, whereas at home this was much higher (Fulton et al, 2016). However, if this can be provided in settings which are less than ideal, then it should be feasible to implement this in the hospital environment.
Exploring the development and use of the bedside resuscitation trolley
In hospital settings, when the need for resuscitation is anticipated, this usually takes place on the resuscitaire; tradition and logistics dictate this is commonly located at the side of the room therefore commanding the need for ICC (Hutchon and Beetles, 2016). For the clinician, this provides advantages, such as having all the equipment readily available in a familiar setup, separation from the family to avoid distraction, and adequate space to work (Thomas et al, 2014). However, the resuscitaire is far from the mother's bedside with clinicians blocking all view and communicating with each other rather than with the parents (Katheria et al, 2017c). This can trigger significant anxiety in the mother as she unable to see or touch her baby (Thomas et al, 2014). In addition, the birth partner is conflicted between following the baby to the resuscitaire or supporting the mother (Sawyer et al, 2015). The distress may be accepted with a belief that separation of the neonate is unavoidable to provide life-saving care.
In a bid to challenge the current practice of ICC, feasibility of bedside resuscitation was assessed by DCC advocates using a standard resuscitaire. Although this was demonstrated successfully, using equipment — which was designed for ‘room-side’ use — identified several practical issues (Hutchon and Bettles, 2016). To overcome these barriers, the LifeStart bedside trolley was devised to allow a more physiological approach to cord clamping (Weeks et al, 2015).
In 2012, feasibility was assessed in a large obstetric unit where 78 babies requiring attendance from an advanced practitioner received treatment (Thomas et al, 2014). ICC was performed in 30% of neonates as the cord was thought too short to reach the trolley. However, this rate declined as the clinician's ability to negotiate more optimal positioning increased. Thomas et al (2014) concluded resuscitation procedures, such as airway management, intubation, and cardiac compressions were safely provided with no adverse effects. Mean gestation in the cohort was 34 weeks, demonstrating suitability for both healthy and compromised neonates. As a secondary evaluation, a semi-structured interview was conducted where the views of 20 clinicians were assessed (Thomas et al, 2014). Despite initial concerns about providing emergency care in such close proximity to the parents, this was mostly expressed by inexperienced clinicians; confidence seemed to increase with use (Thomas et al, 2014; Batey et al, 2017). As use of the trolley continued, overall, 88% reported an improved experience. Interestingly, communication with the parents during resuscitation also improved (Yoxall et al, 2015). However, integration of the trolley introduced a culture change for all clinicians involved, highlighting a need for multidisciplinary training (Thomas et al, 2014).
Neonatal resuscitation is commonly performed by neonatal staff, whose sole focus of care concerns the neonate, therefore separation allows a clear assessment (Katheria et al, 2017b). Midwives offer a more holistic approach, with an aim to prevent separation at birth (Meroz and Gesser-Edelsburg, 2015). But when resuscitation is unexpected, and the paediatrician is not yet present, midwives are still hesitant to initiate care at the bedside, citing hospital culture, and lack of confidence and training as barriers (Fulton et al, 2016). Hollins and Bull (2008) state that midwives are ‘conformers', which may create a personal conflict between their duty to work as an autonomous evidence-based practitioner and the demand to adhere to hospital protocols. This acquiescence is fuelled by fears of adverse outcomes, conflict and litigation (Kirkham, 2011). This could be viewed as a significant barrier which may prevent the midwife from providing holistic care.
Implementation of bedside resuscitation commands the support of all clinicians involved in the provision of care. Conflict arises when more complicated situations are encountered, such as multiple birth or maternal compromise (Reed, 2016). As a principle, any resuscitation should take place with delayed cord clamping but there are circumstances where this is physically impossible.
Currently, in the UK, the bedside trolley has been implemented in Darlington, Wansbeck, Nottingham, Bath and Liverpool Hospital, and Sharp Mary Birth Hospital in the US (Doherty, 2017). More recently, the trolley was used in a trial to assess feasibility of DCC for term neonates without the need for resuscitation (Katheria et al, 2017a). Although this was a pilot trial, DCC was provided for up to five minutes in some neonates. These results are encouraging as they not only present some of the first data comparing term neonates, but they also validate use of the trolley which may increase implementation in other units. However, to date, ICC remains current practice when resuscitation is needed, despite strong evidence suggesting the umbilical cord should remain intact and the development of facilities are required to support this.
Family presence during neonatal resuscitation
Despite the growing body of evidence supporting family presence during resuscitation (Tibballs et al, 2012; Bossaert et al, 2015), this remains a contentious issue. The dispute began following efforts to improve bereavement support and a move towards ‘family-centered’ care (Zottoli, 1998). Consequently, very little is known regarding the parent's opinion of witnessing resuscitation (Katheria et al, 2017b) or any psychological effect this may have.
Most partners are present for the moment of birth, and consequently, would also be there in the event of neonatal resuscitation if this takes place in the same room (Harvey and Pattison, 2012). This presence not only provides emotional and physical support for the mother, but also helps to develop intimate bonds which consolidate the family unit (Coutinho et al, 2016). Increasing recognition of these benefits has fuelled a campaign over the last decade to involve partners more readily in maternity services (RCM, 2011), particularly during childbirth. However, while their ‘involvement’ at the birth is welcomed, negative feelings such as anxiety or stress are not (Draper and Ives, 2013). In the event of neonatal resuscitation, this would leave little room to negotiate the extent of their involvement.
In the first study of its kind, Harvey and Pattison (2012) explored the experience of witnessing neonatal resuscitation through 20 semi-structured interviews with first-time fathers. Although this was a relatively small study, it describes mostly negative emotions derived from four themes, suggesting a lack of emotional support from clinicians during the event. For example, most fathers were uncertain of the extent of resuscitation their baby received. This uncertainty was largely influenced by a lack of information from clinicians (predominantly midwives and neonatal nurses), both during and after the resuscitation (Harvey and Pattison, 2012). When questions were raised, fathers felt they were either answered vaguely or avoided, often information was ascertained through non-verbal communication, which could be misinterpreted. It could be considered that this form of detachment may be adopted by clinicians in order to focus their care on mother and baby. However, just as in birth, the fathers' presence during resuscitation is justified and should not be demoted from participant to observer.
Central to this study was a feeling of conflict over whether to stay and support their partner or follow the baby to the resuscitaire (Harvey and Pattinson, 2012). This decision would no doubt be even more difficult if both were compromised. The results denote the importance of the bedside trolley; bringing care to the mother's bedside may not only improve communication but would eradicate the need to separate the family, which may better support parents during this traumatic situation.
Bedside resuscitation—supporting the family unit
The importance of families being present during resuscitation is well recognised, and now standard, for adult and paediatric care (Leung and Chow, 2012; Bossaert et al, 2015). Research has shown that parental presence during paediatric resuscitation helps provide parents with an understanding that all efforts were being made to save their child (Maxton, 2008). Despite this, the same consideration does not exist in maternity care. The previously mentioned study by Yoxall et al (2015) explored parental presence (fathers) during neonatal resuscitation, and, given the emotions described, may indicate that this was a negative event. Therefore, it is of importance to conduct research which compares current practice, against a suggestion of bedside resuscitation in order to ascertain the parents' standpoint on this issue.
Sawyer et al (2015) conducted qualitative research using semi-structured interviews to explore parents' experience of bedside care during neonatal resuscitation. The data identified five themes indicating that, overall, parents valued the opportunity to be involved in the first moments of their baby's life. The close proximity of the trolley allowed parental contact which provided reassurance (Sawyer et al, 2015). This appreciation was also noted by Yoxall et al (2015) as clinicians remarked parents reached out to their baby during resuscitation. Hutchon (2017b) states that immediately following birth, a mother's touch has a calming effect on the neonate which enhances bonding and may improve the physiological transition to extra-uterine life. The importance of touch is also demonstrated by Arnold et al (2013). Parents describe an immediate bond following the first interaction with their preterm babies, however for most, this was in the neonatal unit (Arnold et al, 2013), suggesting a delay in this emotion was experienced after the birth. This illustrates the impact of separation which would be avoided with bedside care. Moreover, for severely compromised neonates, it may provide the only opportunity for parents to see their baby alive.
| 1. Resuscitation of term neonates should take place with an intact umbilical cord |
| 2. Parents should be fully informed of the need and process for delayed cord clamping (DCC) when resuscitation is required |
| 3. Guidelines should be revised to state that resuscitation should be commenced with an intact cord |
| 4. Attention should be focussed on the birthing environment and equipment to support resuscitation with the umbilical cord intact |
| 5. Multidisciplinary training, which includes simulating resuscitation at the bedside, is required to improve confidence and prevent conflict of opinion at birth regarding cord management when resuscitating |
| 6. Midwifery education should include discussion of the impact of cord clamping and current evidence which supports neonatal resuscitation with an intact cord. This will empower future midwives to bring new skills and knowledge into midwifery practice |
| 7. Further research is needed on the long-term outcomes following DCC when resuscitation is required |
There is an assumed belief that taking the baby away is in the parents' best interests to protect them from psychological trauma. Conversely, parents' experiences of the bedside trolley were mostly positive. While most felt anxious as they witnessed their baby being resuscitated, no regret was expressed thereafter, not even in those who witnessed more intensive procedures (Sawyer et al, 2015). However, as all interviews took place before the parents were discharged, it is possible that with time, opinions and feelings may change as they reflect back on their experience. Although the long-term effects were not analysed, in a recent literature review of paternal presence during resuscitation, Davidson et al (2017) found that families who did not witness resuscitation had significantly higher depression and post-traumatic stress-related symptoms of up to one year after the event than those who did. It could be assumed that this outcome would be the same for parents who witness resuscitation at birth, however, more studies are needed to determine this outcome.
Conclusion
There is a clear indication that resuscitation of term neonates should take place wherever possible with an intact umbilical cord (Mercer and Erickson-Owens, 2012; Bhatt et al, 2013; Hutchon and Bettles, 2016; Uwins and Hutchon, 2014; Katheria et al, 2017a). This does not require a new approach to resuscitation but is one which uses the same accepted approach with the adjunct of a delay in clamping and cutting the umbilical cord until resuscitation is achieved (Thomas et al, 2014; Hutchon and Bettles, 2016). This will be of particular importance for midwives, providing further opportunity to reflect on their current practice, revisit clinical guidelines, and advocate maternity care which is evidence-based (Fulton et al, 2016). While it is noted this change may pose a significant challenge in clinical practice, ICC can no longer be justified as a ‘normal’ intervention and should be challenged.
Nature's wisdom provides a life-line to support the neonate's physiological transition into the world. It is suggested that this process should not be interrupted through compromising the umbilical cord; the midwife should support the neonate's individual competencies by leaving the cord intact. Not only does this provide a vital source of oxygen-rich blood, but this facilitates holistic care as the focus is switched from the need of separation, to promotion of the family unit with lifelong benefit for all.


