In partnership with the editors for the Fundamentals of Maternal Anatomy and Physiology (Peate and Leader, 2024), and Fundamentals of Maternal Pathophysiology (Leader and Peate, 2024), this series provides an evidence-based summary of the cardiac, respiratory and endocrine systems. This first article explores the maternal cardiovascular system, outlining the anatomy and key physiological adaptations in pregnancy, and summarising the key pathophysiological conditions that may occur.
The heart is contained in the thoracic cavity above the diaphragm, enclosed in the pericardial cavity. The apex of the organ is in a lower left position in comparison to the base of the heart at an upper central position. It functions to pump blood throughout the body, has its own circulation system and a cardiac cycle, continuously contracting and relaxing in synchronicity.
Structures and blood flow of the heart
The heart is a hollow muscled organ. The specialised cardiac muscle consists of three layers: the pericardium, myocardium and endocardium. The pericardium is a thin fibrous sheath surrounding the heart. Its rigidity and non-distensible function protects the heart from enlargement and overfilling, anchoring it to the mediastinum. Pericardial fluid lubricates and protects the constantly moving heart from friction during movement. The myocardium, the middle layer, forms most of the heart muscle, comprising elongated, striated muscle cells (myocytes). These cells are specialised, enabling them to work as a single unit while retaining their separate states. As electrical impulses run through the myocardium, the unique myocardial fibres collectively produce the contractions of the heart. The endocardium, an inner layer of cells, lines the heart chambers, assisting with the unhindered flow of blood.
The chambers of the heart
The four chambers of the heart are divided into the left and right side (Figure 1). A muscular interatrial and interventricular septum separates the left and right sides, providing a barrier between oxygenated and deoxygenated circulating blood as it flows through the heart.
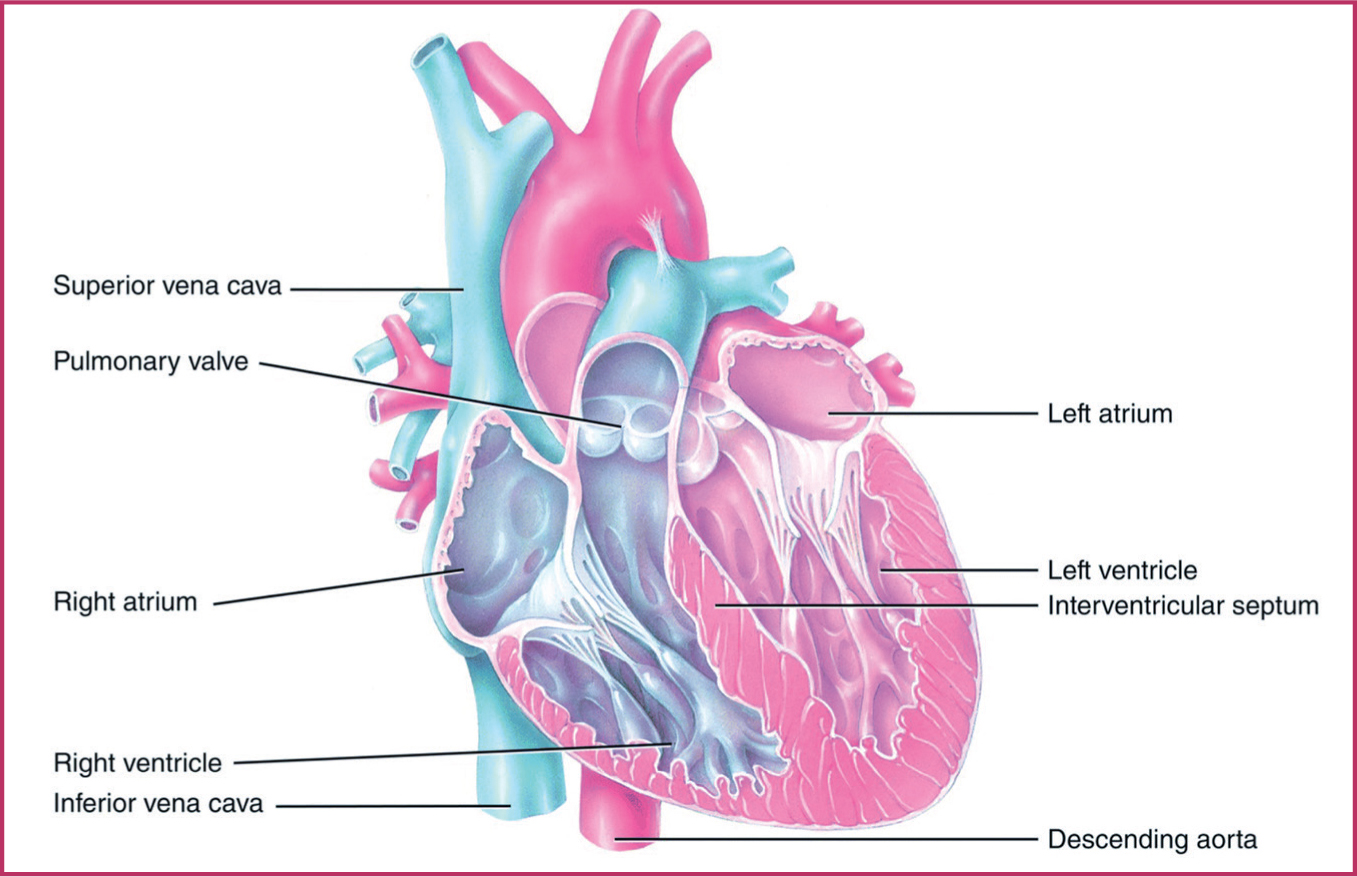
The ventricles have thicker muscular walls. The left ventricle is thicker than the right, and powerfully pumps oxygenated blood into the aortic arch to supply the body with oxygenated blood. The right ventricle has a thinner muscular wall that pumps deoxygenated blood through the pulmonary circuit to the pulmonary artery and the lungs for reoxygenation. The atria act as reservoirs to fill the ventricles.
The direction of blood flow is assisted by valves divided into two categories: the atrioventricular valves and the semilunar valves. Atrioventricular valves include the tricuspid and mitral valve, while semilunar valves include the pulmonary and aortic valve. The left bicuspid/mitral valve has two cusps and the right tricuspid valve has three cusps, both directing blood flow into the ventricles, preventing backflow. The pulmonary artery's valve allows blood flow from the right ventricle to flow into the pulmonary artery and to the lungs. The aortic valve allows high pressure blood flow from the left ventricle into the aorta to oxygenate the whole of the body. When the ventricles relax, the mitral and tricuspid valves open and blood flows into them in preparation for the next contraction. The chordae tendineae, tendinous string like fibrous connective tissue, connect the atrioventricular tricuspid and mitral valves to the heart's papillary muscle, maintaining the position and tension of the valves.
The great vessels of the heart
The five great vessels are large vessels that enter and exit the heart: the superior and inferior vena cava, the pulmonary artery, the pulmonary vein and the aorta (Table 1). Deoxygenated blood flows into the heart through the superior vena cava and the inferior vena cava into the right atrium, then on to the right ventricle, which in turn leads to the pulmonary artery and lungs for reoxygenation. The oxygen-rich blood returns through the pulmonary veins into the left atrium, through to the left ventricle and into the aorta.
| Vessel | Function |
|---|---|
| Superior vena cava | Returns circulating deoxygenated blood from the arms, head, neck, thoracic organs (main organs of respiration and circulation) into the right atrium |
| Inferior vena cava | Returns circulating deoxygenated blood from the body into the right atrium |
| Pulmonary artery | Divides to the left and right, taking deoxygenated blood from the right ventricle to the lungs |
| Pulmonary vein | Returns oxygenated blood from the lungs to the left atrium |
| Aorta | Three-layered vessel: intima, media and externa. Oxygenated blood circulates from the left ventricle through the aorta to supply the whole body |
Cardiac conducting system
The heart's conducting system has electrical impulses that run through the myocardium, allowing contraction of the myocardial fibres and the four chambers to contract rhythmically. The heartbeat initiates at the sinoatrial node, situated at the top of the right atrium setting the rate of contraction. The electrical impulse travels to the atrioventricular node in the bundle of His, a fibrous branch in the intraventricular septum, dividing into two bundle branches to the left and right in the muscular interventricular septum. These supply electrical activity to the left and right ventricles. The electrical impulses are distributed through the Purkinje fibres, located beneath the endocardium. They quickly conduct impulses, enabling synchronised contractions of the ventricles.
Nerve supply
The heart's conducting system ensures the contractions of the heart are continuous, but are influenced by the nerve supply. The autonomic nerve supply unconsciously regulates heart rate, beginning in the brain stem and divided into sympathetic and parasympathetic nerves. The sympathetic nerves arise from the cervical ganglia and prepare the body for a ‘fight and flight’ response by increasing heart rate. The parasympathetic nerve originates in the medulla oblongata and extends throughout the vagus nerve system. It works when the body is at rest to slow down the heart rate. Heart rates are affected directly by internal and external factors. For women during pregnancy, these may include maternal age, circulating blood volume, pre-existing heart disease, medications, stress, smoking, recreational drug use or exercise.
The cardiac cycle
The cardiac cycle is one completed heartbeat from its initiation to its completion. The cardiac muscle can depolarise (cellular excitation), which leads to a contraction of the muscle cells. This includes diastole (relaxation), systole, (contraction) and pause. The frequency of the cycle is measured in beats per minute. The sinoatrial node initiates the heartbeat, with the discrete elements of the heartbeat shown in Figure 2.
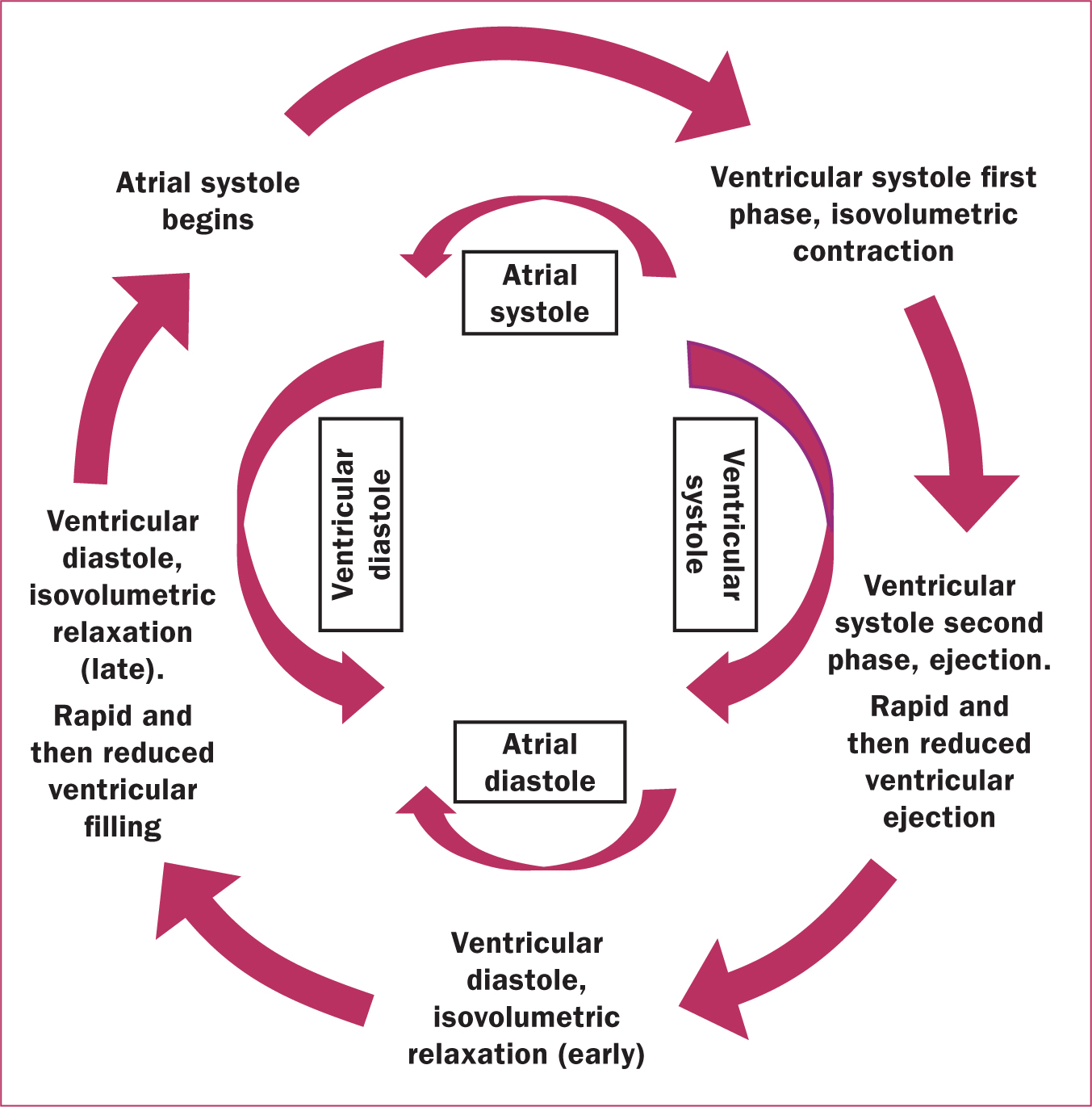
During the cardiac cycle, two sounds can be discerned with each heartbeat and are audible with a stethoscope. Both occur with the closure of the cardiac valves: the first heart sounds are the closure of the mitral and tricuspid valves, and the second sounds are the closure of the aortic and pulmonary valves. These two heart sounds create the ‘lub-dub’ sound heard between pauses.
Pregnancy and childbirth: the maternal cardiac system
Pregnancy is associated with significant changes in the cardiovascular system to meet the increased demands of the mother and fetus and ensure adequate uteroplacental circulation for fetal development. Without these adaptations, morbidity for both mother and fetus can occur.
Cardiac output is the volume of blood pumped by the heart in 1 minute. Stroke volume is the volume of blood pumped by the heart with each beat. Cardiac output can be calculated by multiplying stroke volume by heart rate in beats per minute. Cardiac output increases throughout pregnancy, with the sharpest rise occurring at the start of the first trimester, initially mediated by an increase in stroke volume. By 24 weeks, the increase in cardiac output can be up to 45% compared to a non-pregnant woman (Sanghavi and Rutherford, 2014). During labour, cardiac output increases by an additional 30%, but by 6 weeks postpartum, output will have returned to normal levels (Meah et al, 2016).
The increased stroke volume is a consequence of various adaptations in pregnancy: the left ventricular wall muscle mass increases, leading to increased blood volume in the ventricles prior to systole (end-diastolic volume) and increased myocardial contractility; the heart rate gradually increases in pregnancy by approximately 10–20 beats per minute, reaching higher levels in the third trimester (Nelson-Piercy, 2020). The pregnant uterus compresses the inferior vena cava in a supine (lying back) position, which reduces venous return to the heart. This will lead to reduced cardiac output and so it is important that in emergency situations, or in situations where women may lie flat for prolonged periods (such as during an ultrasound scan), that women are cared for on a lateral tilt.
There is a decrease in blood pressure in the first trimester, usually around the time of the antenatal booking appointment. Hence blood pressure recorded at booking may be lower than preconception values. Arterial pressures begin to increase during the third trimester and return to pre-conception levels postpartum. Blood pressure is affected by cardiac output, circulating blood volume, the elasticity of the vessel walls and systemic vascular resistance (the pressure that the peripheral circulation exerts against the blood pumping from the left ventricle). A decrease in systemic vascular resistance will lead to increased perfusion of peripheral tissues and increased venous flow back to the right ventricle. During pregnancy, there is an increase in peripheral vasodilation (relaxation of the blood vessel walls) as a result of higher oestradiol levels. This vasodilation leads to a drop in systemic vascular resistance and as a result there is a reduction in peripheral blood pressure (Soma-Pillay et al, 2016).
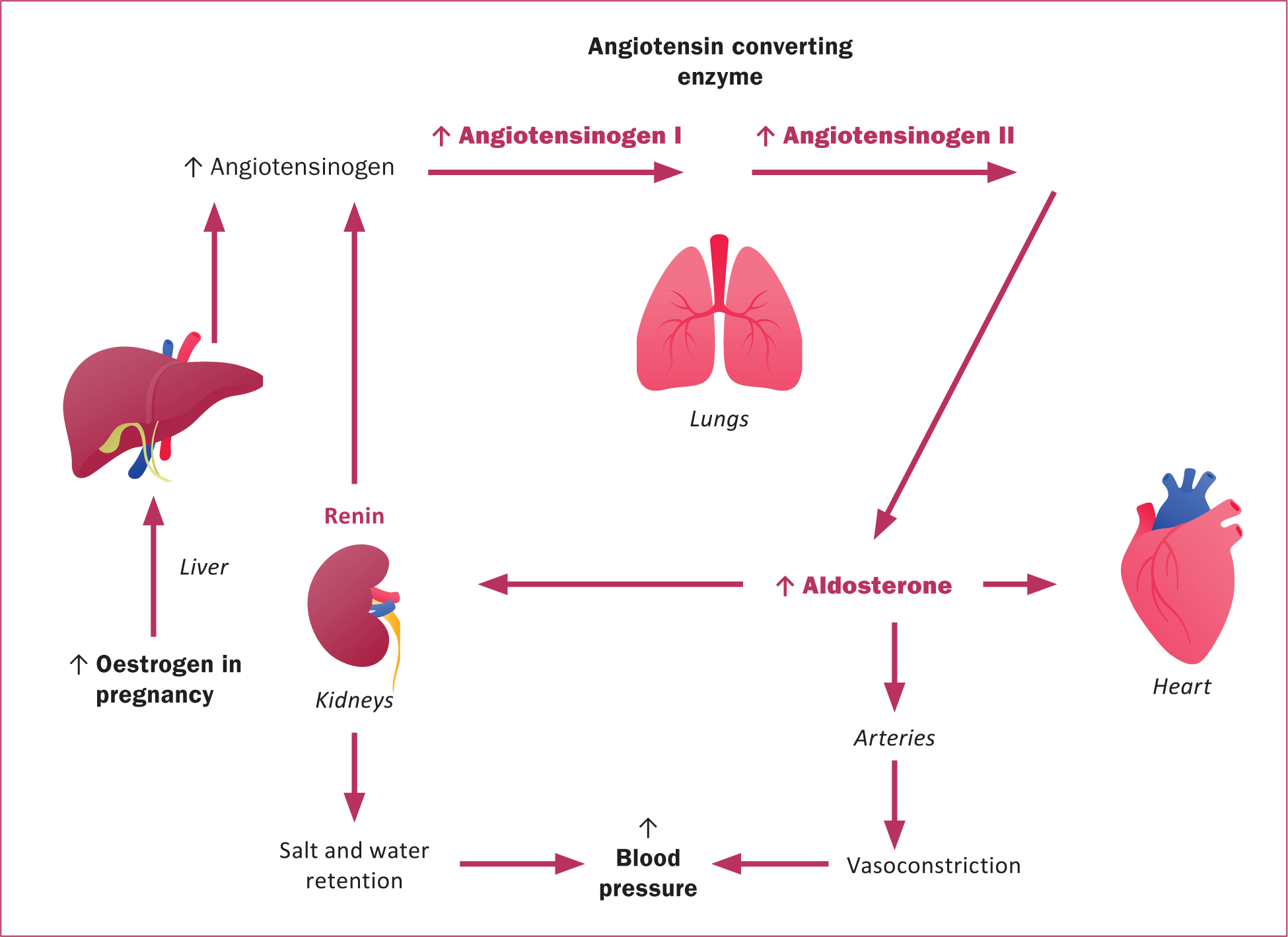
The renin-angiotensin-aldosterone system is a multi-organ pathway that involves regulation of blood pressure, electrolytes, vascular resistance and tone. This pathway is activated in pregnancy, which results in an increase in plasma volume in early pregnancy. As a result of increased oestrogen, angiotensinogen production increases. This leads to salt and water retention, which balances against peripheral vasodilation to maintain an adequate blood pressure in pregnancy (Figure 3) (Soma-Pillay et al, 2016).
Physiology in labour
In labour, contraction of the uterus releases blood back into maternal circulation, leading to increased cardiac preload (the amount of blood that has filled the ventricles when they are relaxed). The autonomic response to pain with contractions results in increased heart rate and blood pressure, and therefore increased cardiac output during labour (Nelson-Piercy, 2020). Following birth, compression of the inferior vena cava is reduced and the uterus remains contracted, releasing blood into the circulation. This increases cardiac output by up to 80% and makes cardiovascular compromise more likely during birth and immediately postpartum. Postnatally, cardiac output will return to normal at around 2 weeks, but overall cardiovascular function may take up to 6–8 weeks to return to normal.
Changes in pregnancy for women with known cardiac impairment
For mothers who have known cardiac impairment, their consultant-led care is usually shared in a combined cardiac obstetric clinic. Midwifery input in these clinics varies across the UK. Many women with medical complexities or comorbidities who are categorised as high-risk attend specialist hospital clinics and may not see a midwife as often as a low-risk mother (Mayer et al, 2020). Ensuring these women have access to midwifery advice and guidance is an essential element of holistic care. For those with existing cardiac disease, their knowledge and understanding of their condition and how it affects their daily living activities may well exceed that of their caregiver. It is important to recognise the mother's autonomy and ownership of her condition.
Congenital heart disease is the most common cardiovascular disease in pregnancy (Siu et al, 2001) m with materal cardiovascular disease estimated to affect 1–4% of pregnanies (Siu et al, 2021). Those with a family history or confirmation of genetic aortopathy or cardiac channelopathy should be referred for cardiac assessment before pregnancy (Knight et al, 2022). Women who are pregnant and have congenital cardiac abnormalities require information to make informed decisions about their care, ongoing surveillance and individualised care planning. Those with existing congenital heart defects should be offered a fetal ultrasound scan to screen for fetal cardiac structural abnormalities. Use of prophylactic antibiotics where endocarditis for cardiac defects (which are not fully closed) may be considered for the intrapartum period, with many women with congenital heart defects treated with prophylactic anticoagulation therapy (heparin/warfarin).
Routine care in the midwifery setting will be complementary to maternal medicine/combined cardiac obstetric clinic appointments, dependent on a woman's needs, and will be individualised. Monitoring of vital signs, regardless of where they present (maternity services, GPs, emergency care etc), requires use of a modified (or maternity) early obstetric warning score (Robbins et al, 2019). This tool identifies deviations from normal specifically for pregnant and postnatal women. However, there is no national standardised tool for this, so slight variations in the reference ranges are currently seen across healthcare providers.
Maternal cardiac disease
The Mothers and Babies: Reducing Risk through Audit and Confidential Enquiries UK reports highlight the importance of a good understanding of cardiovascular presentations in pregnancy, as cardiac disease remains the largest single cause of indirect maternal deaths in the UK (Knight et al, 2021). Maternal mortality rates from indirect cardiac disease accounts for 1.6 per 100 000 maternities (Knight et al, 2022; 2023).
The reports have highlighted the need for good postnatal contraceptive advice and the vital role of pre-pregnancy counselling for high-risk women with pre-existing cardiac disease. Stark inequalities in outcomes are experienced by mothers from Black, Asian and mixed ethnic groups and women who live in the most deprived areas (Knight et al, 2022). The evidence of poorer outcomes for mothers who are at a disadvantage is shaping maternity care. Accurate data collection for this cohort of women contributes to national safer care planning (National Maternity and Perinatal Audit, 2022).
All healthcare professionals caring for women throughout the perinatal period should be aware of common risk factors for heart disease. Considering individual known maternal or familial risk factors should ensure women are counselled to be aware of the signs and symptoms of heart disease. As of 2022, in the UK, services for maternal medicine are delivered via a maternal medicine network. Conditions are categorised into low, medium and high risk and then referred appropriately to the multidisciplinary team, of which midwives are a vital part (Figure 4).

To assess for signs of cardiac disease, a thorough history should be taken. Symptoms to ask about include chest pain, palpitations, breathlessness, light-headedness or fainting (often called pre-syncope and syncope). Orthopnoea is worsening shortness of breath on lying flat and paroxysmal nocturnal dyspnoea describes acute shortness of breath that awakens the patient from sleep; often they will describe waking and gasping for breath (Meng and Arendt, 2021).
Clinical assessment
A pulse oximeter can be used to monitor pulse rate and oxygen saturations, but if cardiac disease is suspected then a peripheral pulse should be palpated manually. Tachycardia or bradycardia may prompt further investigation. Oxygen saturation is the percentage of oxygenated haemoglobin in the blood, and you would expect a healthy woman to have an oxygen saturation of more than 95% in air. Measured blood pressure is necessary at each antenatal visit and in labour. Blood pressure should be recorded in the sitting or left lateral recumbent position (Kinsella, 2006), and the cuff size should be correctly fitted to the woman's upper arm. Respiratory rate is measured ideally when the patient is at rest and is the number of breaths taken per minute. Table 2 summarises normal ranges for basic observations and changes expected in pregnancy.
| Observation | Normal range | Changes in pregnancy |
|---|---|---|
| Heart rate (beats per minute) | 60–90 | Increased by 10–20 |
| Blood pressure (mmHg) | 90/60–140/90 | Decreased by 15–20 in first to second trimester |
| Respiratory rate (breaths per minute) | 12–20 | Unchanged. The volume of air delivered to the lungs in each breath (tidal volume) increases to meet increased oxygen demand |
| Oxygen saturation (%) | 95–100 on room air | Unchanged |
Investigations
Those with suspected cardiac conditions or known cardiac conditions will likely need further investigation. A 12-lead electrocardiogram is usually an initial baseline investigation and can be done at the bedside. The electrical activity of the heart is recorded and this can detect abnormal rhythms, conduction problems, signs of ischemia or strain on the heart muscle. Echocardiography, an ultrasound scan performed by a trained professional, should be requested to investigate for structural abnormalities.
Acute presentations in pregnancy
Women may describe palpitations, a sensation of the heart fluttering or pounding. This can be a common symptom and, if self-limiting and short lasting, in a healthy woman may not be of any concern. However, this can be a symptom of an arrhythmia, heart failure or structural heart disease. Non-cardiac differential diagnoses for palpitations include anaemia, hyperthyroidism, electrolyte disturbance, hypovolaemia or a panic attack. Breathlessness could be a symptom of a range of a serious conditions, but may also be benign in pregnancy. Pregnancy results in increased oxygen demand and tidal volume (the volume of air moved in or out of the lungs in each breath) as well as diaphragmatic elevation, all contributing to this symptom (Soma-Pillay, 2016). Pulmonary oedema occurs because of accumulation of fluid in the lung spaces as a result of ineffective pumping of the heart, and will typically lead to pink frothy sputum, orthopnoea, paroxysmal nocturnal dyspnoea and exertional dyspnoea. When any woman describes chest pain, it is important to gain a thorough history of the onset, character and radiation of the pain and consider if an emergency referral is needed.
Pre-existing cardiac disease
Pre-existing medical conditions and conditions arising in pregnancy result in higher risk pregnancies, and therefore higher morbidity and mortality. The type of cardiac disease, symptoms and severity may all determine how the woman will tolerate pregnancy. Some conditions would be classified as low risk and so may need minimal specialist input in contrast to higher-risk conditions, which require more specialised and closer monitoring.
Mitral stenosis occurs when the mitral valve becomes narrowed, obstructing the blood flowing between the left atrium and left ventricle. Those with mitral stenosis may have symptoms relating to pulmonary oedema including orthopnoea, dyspnoea, postural nocturnal dyspnoea or cough. Aortic stenosis is mostly caused by congenital bicuspid valve disease, and in addition, rheumatic heart disease is also a contributing cause (Regitz-Zagrosek et al, 2018). Symptoms of aortic stenosis are classically angina, breathlessness and syncope.
Both aortic and mitral regurgitation are generally well tolerated in pregnancy. Aortic regurgitation occurs when the aortic valve does not shut completely, leading to backflow of blood into the left ventricle. In mitral regurgitation, there is backflow of blood from the left ventricle to the atrium as a result of incomplete closure of the mitral valve. This can be caused by mitral valve prolapse, which occurs more commonly in young women but is often mild. There are two types of valve replacements: mechanical heart valves or tissue heart valves. Those with mechanical heart valves require lifelong anticoagulation because of the risk of blood clots forming at the valve, which can result in clotting events in the brain or other organs.
Congenital heart disease refers to heart disease that is present from birth. For women, these can encompass a spectrum of diseases of varying severity. Mortality rates from such conditions have declined in recent years, owing to improvements in paediatric care (Nasr et al, 2021). With early detection and appropriate care, these pregnancies can be managed safely and with good outcomes. There are a wide variety of congenital heart conditions with the commoner lesions now explored (Nelson-Piercy, 2020).
An atrial septal defect is a communication between the two atria of the heart from a hole in the dividing septum. It is the most prevalent congenital cardiac defect in the adult population and is usually unproblematic in pregnancy. A ventricular septal defect is an opening in the interventricular septum, which separates both ventricles. It allows blood to pass from the left to the right ventricle and so allows for mixing of oxygenated blood back into the right side of the heart. The heart may have to work harder to pump blood into the systemic circulation. The majority of ventricular septal defects are surgically repaired at birth and so are usually well tolerated in pregnancy.
In most cases, a patent ductus arteriosus either closes soon after birth or is surgically corrected in childhood. The defect involves communication between the aorta and pulmonary artery, present in-utero, remaining open after birth. In most cases a persisting patent ductus arteriosus would be well tolerated (Nelson-Piercy, 2020), however, large cases have the potential for congestive cardiac failure as a result of flow of blood from the left to the right side of the heart (Zhang et al, 2021). In an atrio-ventricular septal defect, there are holes between the right and left side of the heart. In the case of a complete atrio-ventricular septal defect, there is one big hole in the middle of the heart, allowing for mixing of blood between all four chambers. Atrio-ventricular septal defects have a significant association with trisomy 21 and are usually repaired in childhood. Some people may be born with a narrowing of the aorta called aortic coarctation. This would usually be surgically corrected at birth; however, if the narrowing is mild, it may not be detected in childhood. They may present with shortness of breath or raised blood pressure and would typically have higher blood pressure readings in the arms than the legs.
Cardiomyopathies describe illnesses involving the heart muscle, often leading to serious problems with the heart's pumping action and potential heart failure. Examples of cardiomyopathy include hypertrophic cardiomyopathy, dilated cardiomyopathy and peripartum cardiomyopathy. Hypertrophic cardiomyopathy is defined by a non-dilated left ventricular hypertrophy characteristically involving the septum (Antunes and Scudeler, 2020). It has a strong genetic association and 70% of cases are autosomal dominant (Nelson-Piercy, 2020). Dilated cardiomyopathy groups together conditions that result in left ventricular dilatation and dysfunction. Causes of dilated cardiomyopathy range from infection, drug-induced, secondary to ischaemic injury or idiopathic. Idiopathic causes can be hereditary in 20–35% of cases (Ware et al, 2016). Peripartum cardiomyopathy relates to heart failure towards the end of pregnancy or postnatally, where other causes have been excluded (Nelson-Piercy, 2020). The true cause is unknown, but the condition will present with left ventricular systolic dysfunction. The maternal mortality rate is 9–15%, with peripartum cardiomyopathy accounting for 20% of cardiac maternal deaths in the UK (Nelson-Piercy, 2020). Women who have multiple pregnancies, are of an advanced maternal age, are Afro-Caribbean or are multiparous may have a slight predisposition to the condition. The prognosis of peripartum cardiomyopathy is linked to the return to normal left ventricular size and function at 6 months postnatally and those with persisting cardiac dysfunction have a significant mortality risk in future pregnancies because of the risk of recurrence and heart failure.
Acquired heart disease in pregnancy
Acquired heart disease, such as cardiomyopathy, is a leading cause of maternal morbidity and mortality (Park et al, 2021). The changing physiology in pregnancy may reveal de-novo cardiac disease in some patients. Although heart attacks are more common in an older population, cardiac ischaemia can still occur in pregnancy. Cardiac ischaemia occurs when there is restricted blood flow through the blood vessels that supply the heart (coronary arteries), which results in poor oxygen supplies and injury to the heart muscle (myocardium). Some women will present for the first time in pregnancy with an arrhythmia.
Acute coronary syndrome relates to a group of conditions resulting in cardiac ischemia or infarction as a result of reduced perfusion of the coronary arteries. The diagnostic criteria outside of pregnancy apply to pregnant women and include presence of chest pain, electrocardiogram changes and dynamic changes in cardiac enzymes (troponin I). Accumulation of plaque on the coronary artery walls (atherosclerosis) is the most common cause for acute coronary syndrome outside of pregnancy. In pregnancy, other causes may be seen more frequently, including coronary artery dissection (tear in the vessel wall) (Nelson-Piercy, 2020). The most common disease process that causes acute coronary syndrome in pregnancy, usually in the third trimester and beyond, is spontaneous coronary artery dissection followed by atherosclerosis (James et al, 2006).
Aortic dissection can be life threatening. A tear occurs in the medial layer of the aortic vessel wall and blood leaks between the layers. The presentation can vary, but there is typically severe chest pain of rapid onset with radiation to the upper back. There may be raised systolic blood pressure and blood pressure in each arm may be different. A dissection more commonly occurs in the ascending aorta, but symptoms may depend on the blood supply affected. It occurs more commonly in the third trimester and postnatal period (Regitz-Zagrosek et al, 2018). Pregnancy is itself a predisposing factor (Nelson-Piercy, 2020).
Abnormal heart rhythms (arrhythmias) are detected by electrocardiogram and are caused by an issue with the cardiac conduction system. If this system does not work in a synchronised way, cardiac muscle contraction will not be co-ordinated, resulting in arrhythmia. Common types of arrhythmias include atrial fibrillation, atrial flutter, supraventricular tachycardia and heart blocks. These abnormal rhythms can be fast (tachyarrhythmia) or slow (bradyarrhythmia). They may be physiological for some patients but should still prompt investigation as they can, on occasion, be pathological in origin. Arrhythmias may present with palpitations, light headedness, shortness of breath or fatigue and are diagnosed by electrocardiogram.
Care for women with cardiac conditions
Women can be referred for pre-pregnancy counselling through local maternal medicine networks. This has the goal of defining the risks of the condition on a pregnancy and detailing the risks of a pregnancy on the heart condition. Some medications may not be safe for the woman to stop and so the impact of continuing treatments that could impact fetal outcome needs to be discussed. It is vital to consider the woman's hopes and wishes and ensure a joint plan is made going forward.
Antenatally, women should book their pregnancy through their local primary care service and, if they have a cardiac history, should be referred within the maternal medicine network to establish an antenatal plan for care in the most appropriate setting. The woman may need monitoring or investigations in the first trimester. It is also an opportunity to counsel on the impact of pregnancy on their health and that of the fetus. Observations should be monitored at each antenatal appointment and escalated to the medical team if abnormal. Depending on their condition, they should be seen at least in every trimester in a consultant clinic, with some cardiac conditions requiring more frequent input and investigations. If there is risk of cardiac decompensation, then third trimester growth scans will be arranged to monitor for signs of fetal growth restriction because of a potential risk of compromised uteroplacental perfusion. In some cases, more urgent referral to secondary care and maternal medicine services may be required and a plan for early birth may be needed. Pre-existing cardiac disease is a risk factor for other obstetric complications, including postpartum haemorrhage, preeclampsia and preterm labour.
Care in labour and timing of birth will depend on several other factors, including maternal morbidity, fetal wellbeing and maturity, and cervical assessment. In patients taking medications such as beta-blockers, or at risk of cardiac decompensation in later pregnancy, induction of labour may be discussed (Cauldwell et al, 2017; Zhang et al, 2021). The method of induction does not routinely need to be modified. However, high doses of misoprostol should be avoided in cardiac patients because of the potential risk of cardiac vasospasm and arrhythmia. Propess and Prostin prostaglandins can be safely used vaginally for induction of labour. Additional care must be given to speed of titration of oxytocin infusions when augmenting labour in high-risk women who physiologically may have difficulty managing large fluid loads.
Mode of birth must be individualised for each woman to consider the underlying cardiac disease, gestation, any background obstetric factors and maternal wishes. In general, a vaginal birth is considered safe because of the reduced risk of bleeding, venous thromboembolism and infection, and the quicker recovery. An elective caesarean may still be recommended for some women, particularly those with severe aortic pathology, pulmonary hypertension or severe heart failure. Care should be taken to avoid hypovolaemia through postpartum haemorrhage and active management of the third stage should be considered. In the event of bleeding, ergometrine should only be used with caution, and avoided in severe cardiac disease, as ergot can cause coronary artery vasospasm, which could lead to ischemic injury to the heart. Prostaglandin F analogues such as carboprost should also be avoided in cardiovascular disease (Regitz-Zagrosek, 2018).
Postnatally, a venous thromboembolism risk assessment should be performed and prophylaxis should be supplied to the patients. Appropriate follow up with the cardiology team should be arranged. Breastfeeding is not contraindicated, but it is important to consider any medications that may be passed via breast milk and their safety profile.
Conclusions
Midwives require a working knowledge of normal physiology and the most common pathophysiology and diseases, in order to provide individualised care to woman. They need to be aware of ways in which the risk of serious morbidity and mortality from cardiac pathology during pregnancy can be reduced. This is particularly pertinent as cardiac disease remains the leading indirect cause of death in pregnancy. Pre-existing cardiac conditions can significantly affect pregnancy and, conversely, pregnancy can have an impact on cardiovascular function in heart disease. A holistic and multidisciplinary approach, considering the woman's wishes, providing psychosocial support and clearly outlining the risks and care plan, will result in the best outcomes for women and babies.


