Many studies have elucidated the relationship between exposure to environmental tobacco smoke (ETS) and damage to health in children. The World Health Organization (WHO) (1999) performed a large-scale systematic review concerning the effects of exposure to ETS on infant health, and indicated that the risk of diseases of the lower respiratory tract increased 1.7 times in children with mothers who smoked, and was 1.3 times higher in children living with smoking fathers than in children not exposed to ETS. Furthermore, the risk of acute or chronic otorhinological diseases increased by 1.2%–1.4% in children living with two smoking parents compared to children living with non-smoking parents, and the risk of sudden infant death syndrome was five times greater in children whose mothers smoked than in children whose mothers did not. Furthermore, childhood exposure to ETS is associated with learning disabilities, behavioural problems, and speech disorders. DiFranza et al (2004) reported that living with two smoking parents increased the risk of paediatric asthma associated with exposure to ETS by 1.37%, and that exposure to ETS was associated with behavioural problems, cognitive impairment, and increased likelihood of smoking during adolescence. The WHO (2007) claims that ‘smoke-free policies protect health; where they are introduced, exposure to second-hand smoke falls and health improves’. Therefore, promoting smoke-free environments is essential to protect infant health.
In 2003, the WHO Framework Convention on Tobacco Control (FCTC) was adopted in Geneva, and as of 2012, 176 states had signed the treaty (WHO, 2012). After ratifying this treaty, many countries created guidelines and prohibited smoking in public places. Japan signed the FCTC (Ministry of Foreign Affairs of Japan, 2011) to create anti-ETS ordinances for the prevention of exposure to second-hand smoke, and penalties have been sanctioned by various prefectures and wards, restricting smoking in public places. However, in Japan, smoking in public places is not prohibited by law. It differs from 49 other countries, including the UK, which prohibits smoking in all public places (WHO, 2015). In Japan, more than 30% of individuals experience passive smoking in restaurants, amusement facilities, and workplaces (Ministry of Health, Labour and Welfare, 2013). Furthermore, there are no restrictions concerning smoking in the home. A high incidence of smoking (approximately 40%) was observed among fathers in the fourth and fifth decades of life (Ministry of Health, Labour and Welfare, 2012). These factors greatly influence the incidence of exposure to ETS among children in the home. Kubo et al (2012) reported that the increased ETS exposure of pregnant women living with a smoker resulted in significantly higher cotinine levels in neonates soon after birth than in those born to mothers living with non-smokers, indicating that exposure to ETS in pregnant women affects the fetus. However, no assessment of the status of exposure to ETS among infants and mothers has been performed in other age groups.
Objective assessments of the exposure to ETS, other than the evaluation of the smoking rate, include the measurement of chemical substances (biomarkers) absorbed into the body through inhalation. The measurement of nicotine exposure is the most popular method for analysing the biomarkers absorbed by the body during exposure to ETS. Among the biomarkers, the measurement of cotinine, a nicotine metabolite, is the most common (United States Department of Health and Human Services (USDHHS), 2006). Because the half-life of cotinine (16–19 hours) is longer than that of nicotine (1–3 hours), cotinine has been indicated as a biomarker to assess exposure to ETS (Jarvis et al, 1988). Moreover, cotinine is very specific because it is not usually present in the body without exposure to tobacco smoke, and sensitive because it can be measured at low concentrations (International Union Against Cancer, 2008). Both nicotine and cotinine can be measured in various body fluids, but most studies on exposure to ETS have used blood, urine, saliva, or hair samples (USDHHS, 2006). However, blood sample collection is an invasive procedure and difficult to perform in small children.
Bernert et al (2000) have reported that a linear correlation was observed between salivary and blood cotinine concentrations. Therefore, salivary cotinine analysis is a useful, quick, and noninvasive approach to assess exposure to ETS. For this reason, we propose the use of salivary cotinine as an objective indicator of exposure to ETS in infants.
This study aimed to determine the longitudinal variations in exposure to ETS in the home among infants (at 1, 3, 6, and 10 months of age), and the association between infants' exposure to ETS in their living environment and the presence of cotinine.
Methods
Research design and subjects
This study used a prospective cohort design. The mothers were in the late period of pregnancy (36–40 weeks) and were subjected to physical examinations in a general hospital in the Japanese capital area. When they came to the hospital for prenatal examinations, the examiners explained the study aims and methods. All individuals who enrolled in the study were requested to sign an informed consent document.
The inclusion criteria were:
The following items were explained orally and in writing to the subjects:
The present study was approved by the Ethical Committee of the university (protocol number 10040) and the ethical and medical review board of the maternity facility (protocol number 09-12-1), where the study was conducted.
Salivary sample collection and analysis
Saliva samples from the infants and mothers who consented to participate in the study were collected in a maternity facility in four distinct periods (1, 3, 6, and 10 months after birth). To avoid the influence of residual milk in the oral cavity after breastfeeding, saliva samples (0.5–1.0 ml) were collected 30 minutes or later after breastfeeding for infants, and 30 minutes or later after meals for mothers. To ensure privacy, saliva samples were collected in a private patient examination room. Furthermore, we ensured that the subjects' concerns and questions about study methods were addressed.
Saliva samples were collected from infants using a sponge (diameter 10 mm by 25 mm) placed between the gum and cheek or under the tongue in order to absorb enough saliva, which was then transferred to a specialised container and sealed. The saliva-moistened sponge was then centrifuged. Saliva samples were frozen until analysis. Saliva samples from mothers were collected directly via a capillary into a specialised container, which was sealed and frozen for storage. Katsumata et al (2009) compared the two methods (using specialised sponges for saliva collection vs directly flowing saliva) and observed no significant differences in the measured cotinine values.
The cotinine concentrations of all samples were measured by Cosmic Corporation Ltd (Tokyo, Japan). The exposure to ETS in infants and mothers was evaluated by measuring the cotinine levels in saliva samples using an enzyme–linked immunosorbent assay.
Data collection
Data pertaining to the saliva samples from infants and their mothers, and self-administered questionnaires and interviews with the mothers were collected between June 2010 and September 2011 at the general hospital in Japan. We applied questionnaires and interviews about the age of the mother, history of previous childbirth, living with a smoker, and smoking areas in the home.
Data analysis
A McNemer's test was used to compare the presence or absence of cotinine in infants and mothers.
A Cochran's Q test was used to assess the presence of cotinine in infants according to the measurement periods. Risk rates and 95% confidence intervals were used to compare the following variables:
The presence of cotinine was defined as cotinine levels of ≥0.6 ng/ml.
SPSS version 18 was used for statistical analysis.
Results
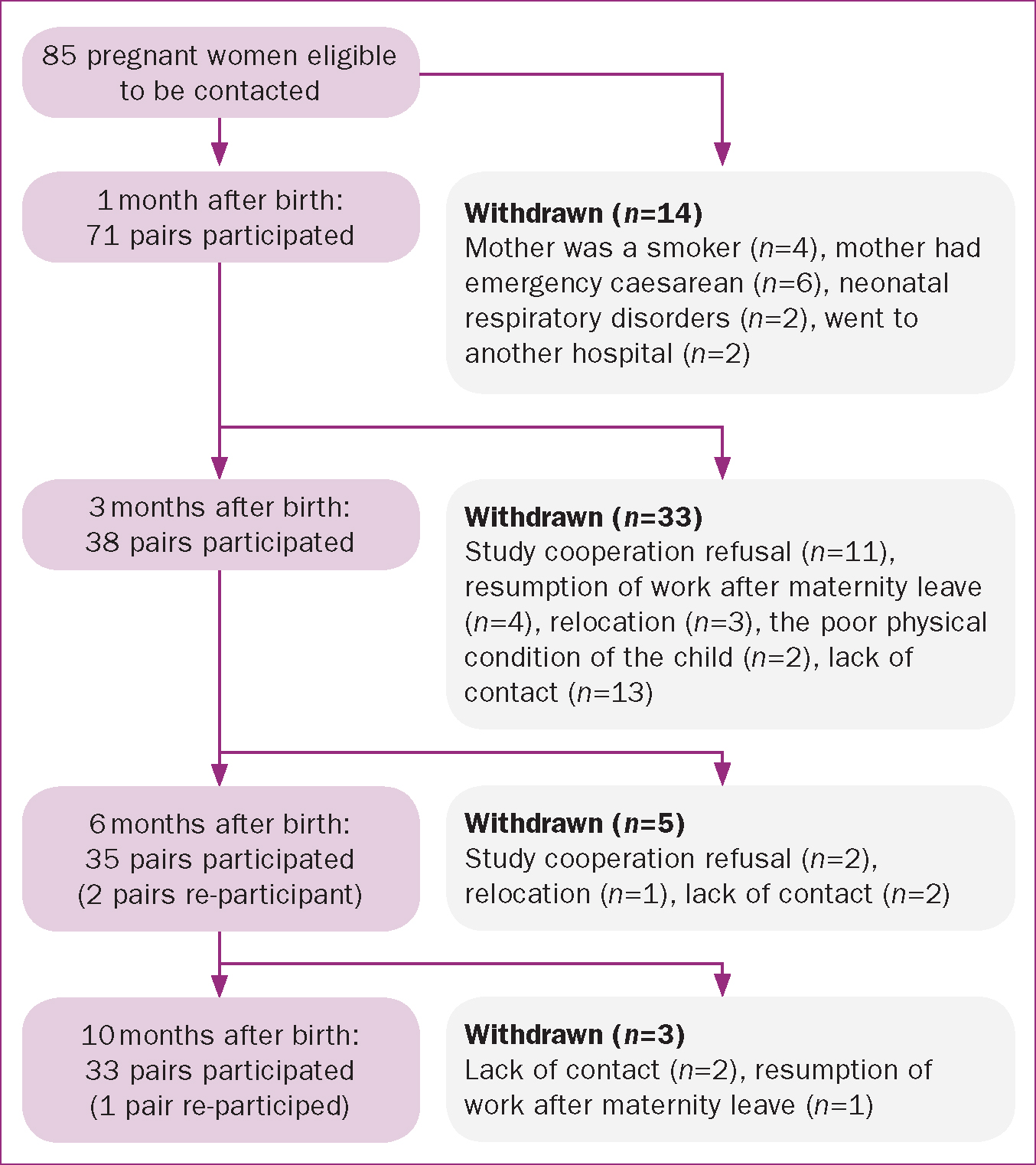
Initially, we obtained the consent of 85 pregnant women to participate in the study. However, the number of participants reduced progressively as follows: 1 month after birth, 71 pairs of mothers and infants remained; 3 months after birth, 38 pairs remained; 6 months after birth, 35 pairs remained; and 10 months after birth, 33 pairs remained. Reasons for withdrawal included emergency caesarean sections, neonatal respiratory disorders at the time of birth, and study cooperation refusal. Two pairs joined the study again 6 months after birth (Figure 1).
Over 70% of the participating mothers were aged 30–34 years (41.3%) or 35–39 years (33.3%). No mothers were aged <20 or >40 years. In total, 49.3% of infants lived with a smoker; of these, the father was usually the family member who smoked (91.9%) (Table 1).
| Items | Number of individuals | % | |
|---|---|---|---|
| Age of the mother | 20–24 years | 4 | 5.6 |
| 25–29 years | 13 | 18.3 | |
| 30–34 years | 29 | 40.8 | |
| 35–39 years | 25 | 35.2 | |
| Primipara or multipara | Primipara | 51 | 71.8 |
| Multipara | 20 | 28.2 | |
| Smoker living in the same household | No | 37 | 52.1 |
| Yes | 34 | 47.9 | |
| Smoker living in the same household (n=37) | Father | 31 | 91.2 |
| Another family member | 4 | 11.8 | |
The cotinine detection rate in infants did not vary among the different measurement periods. Likewise, no significant differences in cotinine detection rate between the different measurement periods were observed in the mothers. At 1 month (P=0.022) and 3 months (P=0.031) after birth, the cotinine detection rate in infants was higher than that of their mothers (Table 2).
| 1 month n=71 | 3 months n=38 | 6 months n=35 | 10 months n=33 | P | |||
|---|---|---|---|---|---|---|---|
| Cotinine detection (%) | Infants | + | 17 (23.9) | 7 (18.4) | 4 (11.4) | 6 (18.2) | a 0.691 |
| – | 54 (76.1) | 31 (81.6) | 31 (88.6) | 27 (81.8) | |||
| Mothers | + | 8(11.3) | 1 (2.6) | 1 (2.9) | 1 (3.0) | a 1.000 | |
| – | 63 (88.7) | 37 (97.4) | 34 (97.1) | 32 (97.0) | |||
| Comparison of infants and their mothers | p | b 0.022 | b 0.031 | b 0.250 | b 0.063 | ||
Notes:
Cochran Q test
McNemer's test
Cotinine detection rates for the 36 pairs (mothers and infants) who remained in the study beyond 1 month and those for the 39 pairs that dropped out after 1 month were compared to investigate whether attrition was related to cotinine detection. A Pearson's chi-square test was used for this analysis. There was no difference in the cotinine detection rate for infants (P=0.956), but there was a significant difference for mothers (P=0.014).
To understand the status of ETS exposure among infants in the home, we compared the following variables:
Comparison between the rates of cotinine detection in infants who lived with a smoker in the household and those who did not revealed that cotinine detection rates are significantly higher for infants living with smokers than in infants living with nonsmokers 1 month, 6 months, and 10 months after birth (Table 3).
| Category | Cotinine detection | 95% Confidence interval | |||||
|---|---|---|---|---|---|---|---|
| Yes | No | Risk ratio | Lower limit | Upper limit | |||
| Living with a smoker in the household | 1 month after birth | Yes | 16 | 17 | 1.890 | 1.352 | 2.643 |
| No | 1 | 37 | |||||
| 3 months after birth | Yes | 4 | 9 | 1.271 | 0.860 | 1.878 | |
| No | 3 | 22 | |||||
| 6 months after birth | Yes | 4 | 8 | 1.500 | 1.005 | 2.238 | |
| No | 0 | 23 | |||||
| 10 months after birth | Yes | 6 | 5 | 2.200 | 1.152 | 4.203 | |
| No | 0 | 22 | |||||
Comparison between the cotinine detection rates for different smoking areas revealed that 1 month after birth the cotinine detection rate was higher in infants living with a family member who smoked on the balcony than in those living with nonsmokers or with a family member who smoked outdoors. Similarly, the cotinine detection rate was higher in infants living with a family member who smoked indoors than in those living with a family member who smoked outdoors (Table 4).
| Category | Cotinine detection | 95% Confidence interval | ||||
|---|---|---|---|---|---|---|
| Yes | No | Risk ratio | Lower limit | Upper limit | ||
| Smoking area | Outdoor | 0 | 9 | 0.946 | 0.876 | 1.022 |
| Non-smoker | 2 | 35 | ||||
| Indoor | 5 | 1 | 5.676 | 0.947 | 34.023 | |
| Non-smoker | 2 | 35 | ||||
| Balcony | 10 | 8 | 2.128 | 1.263 | 3.588 | |
| Non-smoker | 2 | 35 | ||||
| Indoor | 5 | 1 | 6.000 | 1.003 | 35.908 | |
| Outdoor | 0 | 9 | ||||
| Balcony | 10 | 8 | 2.250 | 1.342 | 3.771 | |
| Outdoor | 0 | 9 | ||||
| Indoor | 5 | 1 | 0.375 | 0.058 | 2.414 | |
| Balcony | 10 | 8 | ||||
No significant differences were observed between infants whose family members were nonsmokers compared to those whose family members smoked outdoors or indoors. Similarly, cotinine detection rates for infants whose family members smoked indoors and those whose family members smoked on the balcony did not differ 1 month after birth (Table 4).
There were no differences for the different smoking areas 3 months, 6 months, or 10 months after birth.
Discussion
The results of the present study show that for infants and mothers who are believed to live in similar environments, the cotinine detection rate was significantly higher in infants 1 month after birth. These results may be due to differences in nicotine and cotinine metabolism between infants and adults. In this respect, Dempsey et al (2000) reported no significant differences in the half-life of cotinine in the blood and urine of neonates and adults. However, the half-life of nicotine was three to four times higher in neonates. Nicotine is metabolised in the liver, and because hepatic function in neonates is limited, the metabolism of drugs and other metabolites is delayed (Nishida, 2012). Hence, the half-life of nicotine is longer in infants than in adults. For this reason, a higher rate of cotinine was expected to be found among infants compared to adults.
Becquemin et al (1991) showed that the rate of intranasal adsorption of particles was lower in children aged 5 years and that children were not protected from particle inhalation via the airway. Furthermore, the Air Quality Committee of the Central Environment Council (2009) reported that the amount of particles per surface area unit was not different for children and adults. However, the respiratory rate and respiratory minute volume per surface area of the lungs as well as the risk of particle inhalation seemed to be greater. Therefore, protection against particle inhalation was poorer in children than in adults. Because particle inhalation per surface area levels are higher in children, the results may be similar to those observed for nicotine inhalation.
Our results indicate that compared to adults, infants who cannot move away from tobacco smoke themselves are more severely affected by exposure to ETS. These findings should be communicated to households where infants are present or live nearby. Health and medical institutions are already guiding families regarding the prevention of infant exposure to ETS. However, by further informing these families about the increased susceptibility of infants to ETS exposure compared to adults, it may be possible to increase their awareness of ETS exposure prevention in the home.
The presence of a smoker in the household increased the risk of detecting salivary cotinine in infants 1 month, 6 months, and 10 months after birth, and was associated with exposure to ETS in infants. These results were similar to those of previous studies, which have reported that cotinine values in infants living with a smoker in the household were higher than in infants who were not exposed to ETS (Daly et al, 2001; Seifert et al, 2002; Daly et al, 2010). In the present study, most of the smokers in the household were the infants' fathers (91.2%). Therefore, ETS exposure in the home is often due to the father's smoking habits, and if the father stops smoking, ETS exposure in infants can be eliminated. Moreover, this suggests that midwives need to engage with all family members if they are to ensure optimal health outcomes for women and their offspring.
Cotinine was detected in two infants even though they did not live with smokers. It is possible that they were exposed to tobacco smoke from non-family members because cotinine is usually not detected in individuals who are not exposed to tobacco smoke (International Union Against Cancer, 2008).
Cotinine detection rates were higher in infants whose family members smoked inside the home than in those whose family members smoked outside, and higher in those whose family members smoked on their balcony than in those whose family members smoked outside or did not smoke. Seong et al (2008) measured nicotine and cotinine levels in hair samples of neonates who had been exposed to ETS before birth and reported higher levels in neonates living with a family member who smoked in the home or on the balcony than in those living with a family member who smoked outside. Kubo et al (2012) measured cotinine levels in saliva samples of neonates immediately after birth and reported higher levels in neonates living with a family member who smoked in the home than in those living with a family member who smoked outside. The present study showed similar results for infants.
Many Japanese smokers choose to smoke on their balconies, which are outside their homes. To reduce ETS during infancy, it is necessary to ensure that the inside of the home and the balcony are smoke-free. Furthermore, it is important to create awareness among those who smoke on the balcony about the fact that smoking on the balcony increases ETS exposure among infants, and that it is a risk factor for the development of health problems. Therefore, only complete abstention from smoking will eliminate the effects of ETS exposure among infants.
This study has several limitations. In this cohort study, the number of participants decreased after 3 months of birth. We believe this was because of no differences between cotinine detection rates of infants and mothers, and between infants and family members' smoking areas after 3 months of birth. The findings need to be verified with more subjects. In addition, more infants and mothers may have participated if the researcher paid home visits to collect saliva samples.
Conclusions
Our results revealed that the cotinine detection rate was higher in infants than their mothers despite living in similar environmental conditions. Further, the ETS exposure status of infants was related to living with a smoker in the household, living with a father who smokes, and family members smoking inside the home or on the balcony, as opposed to outside the home.
To prevent ETS exposure among infants, it is important to communicate the fact that infants are more easily affected by exposure to ETS than adults, pro-actively educate fathers concerning the prevention of exposure to ETS, and make the home and balcony smoke-free. Interventions to prevent infant exposure to ETS need to be created and then evaluated for efficacy.
